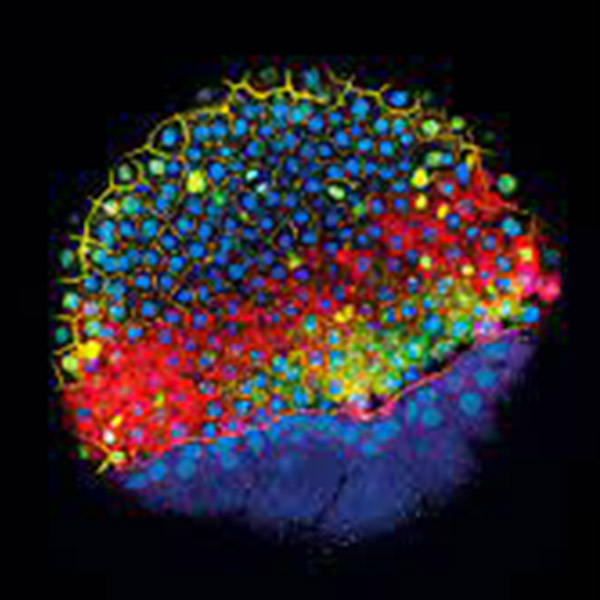
San Francisco clinical surgeon Michael R. Harrison was the first to complete fetal surgery. He served as division chief in pediatric surgery at the Children's Hospital at the University of California, San Francisco for over 20 years. He is often referred to as the father of fetal surgery as it is now used regularly to fix abnormalities or other conditions in unborn babies. He made two attempts to unblock urinary tract obstructions in fetuses in 1981; the second surgery resulted in a healthy baby. Today, many more surgical procedures are available to treat fetuses.
40
years breakthroughs stories
40 years of extraordinary connections
A 40-year journey shaping extraordinary links between elite life science talent and pioneering pharma, biotech and healthcare organisations. Showcasing the revolutionary impact that results from connecting innovative leaders, and celebrating the incredible people behind the growth and advancement of the life science and healthcare sectors around the world.
Adam Aspinall
Adrian Cottrell
William Burns
Bruno Febra
Dr. Carsten von Blohn
Christian Joergensen
Dr. Colton Wong
David Arias Gayo
Malcolm Taylor
Dr. Manfred Kurz
Matthew Lumley
Dr Maziar Assadi Gehr
John Overington
Jordi Clarimon
Nick Page
Nigel Brooksby
Professor Peter Simpson
Jina Swartz
Samantha Nickerson
Sebastian Hallworth
Simon Collis
Tim Watts
Yann Guivarch
Ahmed Al-Derzi
Dr Ian Hudson, OBE
Jasna Knezevic
Pierre van Weperen
Jo-Hanna Krause
John Bolodeoku
Kok Eng Ng
Dr. Kulasiri Gunawardena
Lee Smith
Adam Aspinall, Senior Director, Access & Product Management, Medicines for Malaria Venture | Honorary Fellow, Durham University Business School | Chair, Fight the Fakes Alliance
Adam Aspinall’s experience spans big pharma and small biotech, blockbuster and niche orphan drugs.
With a specific interest in marketing planning, product launches, pharmerging markets, developing junior marketers and business ethics, Mr Aspinall’s is a Chartered Institute of Marketing, director of a palliative care charity and a former director of the Association of Renal Industries.
Chair and founder of the Fight the Fakes Alliance and Senior Director of Access and product management at Medicines for Malaria Venture (MMV), his specialties and areas of therapeutic experiences include scaling-up action to prevent falsified medicines from endangering lives across the globe, Anaesthesia, Pain and Critical Care, Renal Medicine, Oncology, Anti-Infectives, and both primary and secondary care.
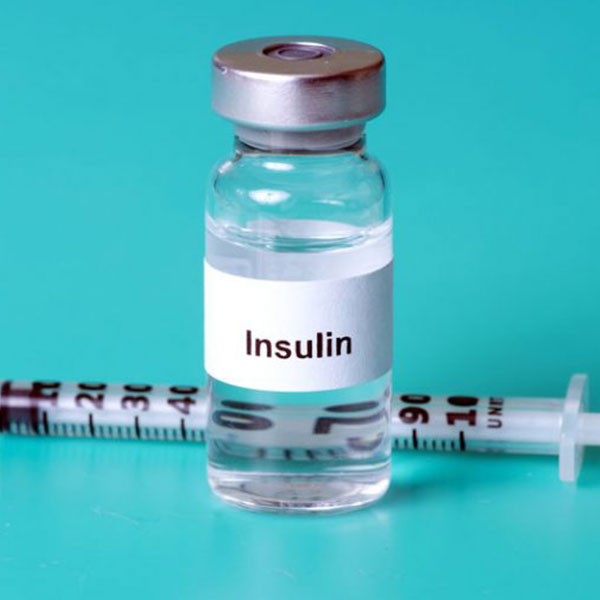
1982 First Genetically Engineered Human Drug - Synthetic Insulin
At a company in the biotech game for over 40 years, Genentech scientist Dennis Kleid helped put the world’s first genetically engineered drug - insulin- on the market for humans. Insulin had been used for the better part of a century to treat patients suffering from Type I diabetes. Historically, insulin had been harvested from animals, and while similar to human insulin, animal insulin has a few significant differences.
Moreover, in 1978, a single pound of insulin required 8000 pounds of pancreas glands from 23,500 animals. This required 56 million animals per year to meet increasing demand in the United States; a synthetic alternative was clearly necessary. This is considered a defining moment of Genentech’s history, as well as a defining moment for the concept of genetically engineered drugs being approved for human consumption.
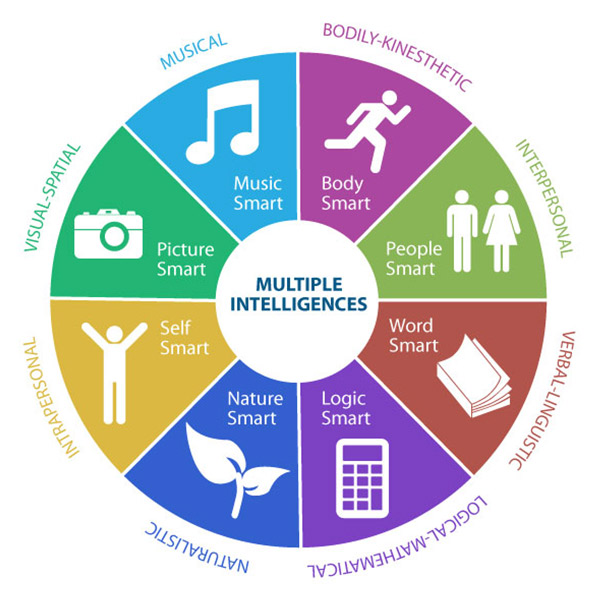
1983 – Howard Gardner proposes the Theory of Multiple Intelligences
The theory of multiple intelligences was first proposed by Howard Gardner in his 1983 book “Frames of Mind”, where he broadens the definition of intelligence and outlines several distinct types of intellectual competencies. Gardner’s eight types of intelligence include: Linguistic, Logical/Mathematical, Spatial, Bodily-Kinesthetic, Musical, Interpersonal, Intrapersonal, and Naturalist. He writes that we may all have these intelligences, but our profile of these may differ individually based on genetics or experience. Gardner’s work highlighted that quantifying human intelligence is an inaccurate way to measure people’s abilities and was hence seminal to the advancement and use of psychometric testing as a practice standard. This soon became a core part of RSA’s due diligence process and has developed and evolved over the years resulting in over 90% of candidates remaining in their role or being promoted 24 months after appointment.
.
1984 - DNA fingerprinting uncovered
University of Leicester geneticist Alec Jeffreys analyzed DNA to discover that certain repetitive patterns are present in all humans but vary in length . This discrepancy was specific to everyone and could be used to link biological samples to out someone’s identity. He coined the term “genetic fingerprinting,” a development which contributed to paternity testing, crime scene analysis, and more.
1985 - The human TP53 gene was cloned in 1984 and the full-length clone in 1985
In 1993, p53 was voted molecule of the year by Science magazine. The human TP53 gene was cloned in 1984 and the full-length clone was completed in 1985. p53 has been described as "the guardian of the genome" because of its role in conserving stability by preventing genome mutation. Sir David Philip Lane is a British immunologist, molecular biologist and cancer researcher. He is currently Chief Scientist of the Agency for Science, Technology and Research (A*STAR) in Singapore, Scientific Director of Ludwig Cancer Research and Chairman of Chugai Pharmabody. He is best known for the discovery of p53, one of the most important tumour suppressor genes. The RSA Group and Sir David Lane have a strong relationship of mutual consultations, and we are collaborating tightly with A*STAR on some of their recent appointments.
Around 1986 (and a couple of years prior), scientists were working on developing a vaccine for Hepatitis B. Previously in 1963, Baruch Blumberg developed a blood-derived vaccine for Hepatitis, which was approved for market in 1981. Once Pablo D. T. Valenzuela was able to create the world’s first recombinant vaccine using yeast cells, primarily Saccharomyces cerevisiae.
This vaccine immediately became the market standard, and the blood-derived vaccine was removed from circulation. By establishing a recombinant process for vaccines, many vaccines we still use today were created, including for diseases such as: HPV, whooping cough, pneumococcal, meningococcal, Haemophilus influenzae type b (Hib), and shingles.
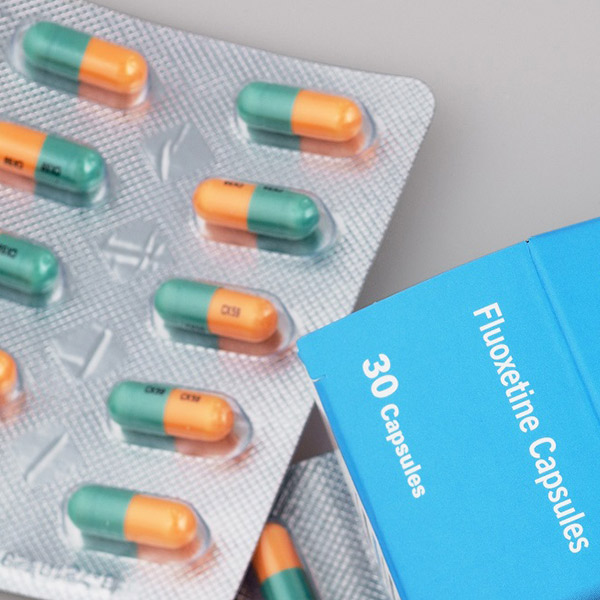
1986 – Eli Lilly revolutionises mental healthcare - releases Fluoxetine, world’s first SSRI drug
Although the compound was synthesised in 1972, Fluoextine took 14 years of clinical development before it reached the Belgian Pharmaceutical market in 1986. A year later, Fluoextine (brand name Prozac) was FDA approved and available in the United States. Discovery occurred at Eli Lilly where Bryan Molloy and Robert Rathburn were attempting to isolate the serotonin reuptake function of monamine oxidase, the common antidepressant drug of the day. Accordingly, Fluoextine and its counterparts are labelled SSRIs (Selective Serotonin Reuptake Inhibitors), as they attempt to regulate the uptake of Serotonin in certain parts of the brain in order to treat mental illnesses such as depression, OCD, bulimia and panic disorder.
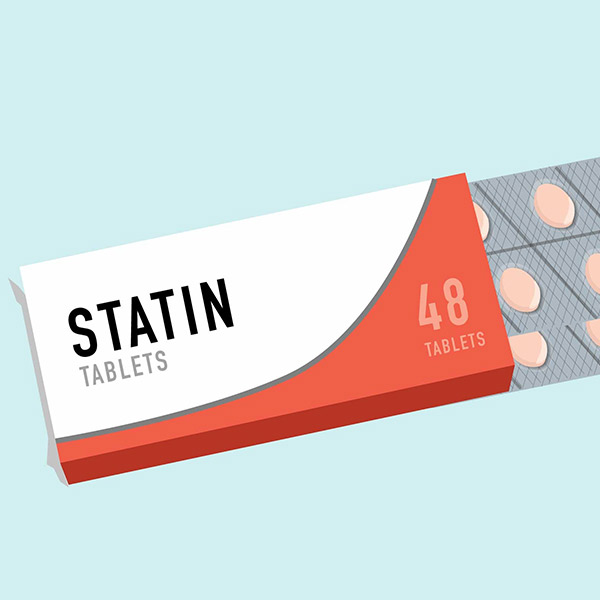
1987 Merck’s statin medication Mevacor gets FDA approval, innovating cardiovascular disease space
With the research developments of the previous decades, in the 70s and 80s it became apparent high concentrations of plasma cholesterol were a major risk factor for the development of coronary heart disease, which led to the search for drugs that could reduce plasma cholesterol. One way to do this is to reduce cholesterol biosynthesis, with the rate-limiting enzyme in the cholesterol biosynthetic pathway HMG-CoA reductase being a natural target. Merck first commercialised lovastatin in 1987 and ever since then 6 statins have been introduced to the market. Today the most popular statin is Pfizer’s atorvastatin. The remarkable safety of statins derives from their unique mechanism of action, making them one of the most widely used drug on the planet. Today, an estimated 30 million people are taking statin and it’s been proven to reduce frequency of heart attacks by 25–30%.
1988 - Patricia Bath patents the Laserphaco Probe, a device for ablating and removing cataract lenses
Bath developed the Laserphaco Probe, a medical device that improves on the use of lasers to remove cataracts and coined the term "Laser phaco" for the process, short for laser PHotoAblative Cataract surgery. Bath first had the idea for this type of device in 1981 but did not apply for a patent until several years later. The device was completed in 1986 after Bath conducted research on lasers in Berlin and patented in 1988, making her the first African-American woman to receive a patent for a medical purpose. The device — which quickly and nearly painlessly dissolves the cataract with a laser, irrigates and cleans the eye and permits the easy insertion of a new lens — is used internationally to treat the disease. Bath has continued to improve the device and has successfully restored vision to people who have been unable to see for decades.
Bath holds five patents in the United States. Three of Bath's five patents relate to the Laserphaco Probe. In 2000, she was granted a patent for a method for using pulsed ultrasound to remove cataracts, and in 2003 a patent for combining laser and ultrasound to remove cataracts.
Though hepatitis C has plagued humans throughout history, the particulars about how the disease works were largely unknown until 1989 . Researchers at the California biotech company Chiron and the Centers for Disease Control and Prevention identified a new virus separate from known forms of hepatitis, a discovery which led to methods for testing individuals and screening blood donations.
1989 – Sir Greg Winter establishes Cambridge Antibody Technology
Sir Greg Winter is one of the biggest names in European biotech. As a Professor of Biochemistry at Cambridge University, he developed phage display technology that became the foundation of his first company in 1989. Sir Greg Winter patented human monoclonal antibodies and every antibody medicine since has been based on his work, with most of the largest selling drugs in the world coming from his IP. The first of Greg Winter’s three antibody-related companies, Cambridge Antibody Technology (CAT), would go on to be the biggest success story in UK biotech after its team discovered the blockbuster therapeutic antibody, Humira, and became part of AstraZeneca in 2006 in a £702M acquisition.
1990 - Human Genome Project
From 1990 to 2003, the Human Genome project succeeded in mapping the human genome with more than 20 thousand genes identified and their genomic loci documented. In 2003, the ENCODE (Encyclopedia of DNA Elements) project kicked off, with the aim of creating a complete list of the functional elements of the human genome, including elements that act at both the protein and the RNA level, including regulatory elements controlling transcription, translation and replication. The 1990s was a period of discovery and proved that the work being done in this field was not only valid, but necessary.
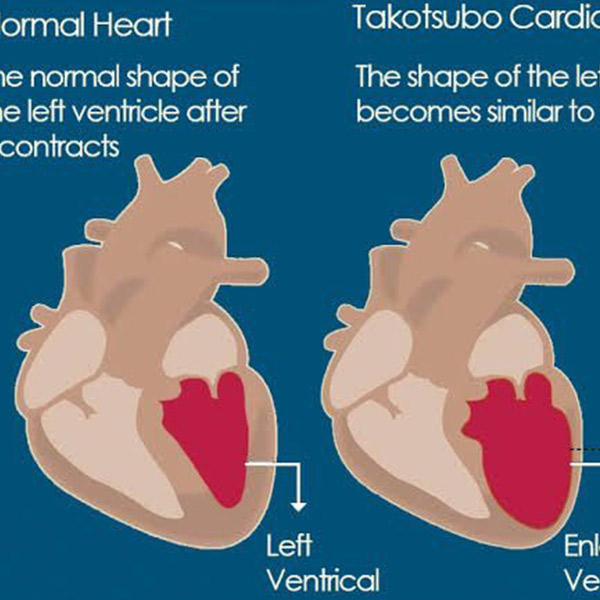
1991 - The first studied case of takotsubo cardiomyopathy
Takotsubo cardiomyopathy is a weakening of the left ventricle, the heart's main pumping chamber, usually as the result of severe emotional or physical stress, such as a sudden illness, the loss of a loved one, a serious accident, or a natural disaster such as an earthquake. That's why the condition is also called stress-induced cardiomyopathy or broken-heart syndrome. The main symptoms are chest pain and shortness of breath. This was first investigated by Sato et al. 1991.
1992 – The Wellcome Trust established the Sanger Institute
The Wellcome Trust established the Sanger Centre in 1992 to undertake the most ambitious project ever attempted in biology, sequencing the human genome. The new facility developed laboratory infrastructure, robotics, team working and computational approaches on a scale unprecedented in life sciences. The Sanger Centre opened in 1993. The genome sequencing facility is named after Fred Sanger, the double Nobel Laureate who devised the method for DNA sequencing used in the Human Genome Project. A modest individual, Sanger reluctantly gives permission for the use of his name. Having accepted, however, he cautions… “it better be good”
In 2000, the first draft of the human genome was announced with the Sanger Centre championing open access to the data and making the largest contribution to the global collaborative endeavour. Genomes began to convert biology into big data science. The subsequently renamed Wellcome Trust Sanger Institute established long term research programmes to explore and apply genome sequences.With support from Wellcome and world-leading facilities, Sanger Institute scientists today continue to undertake research that cannot easily be conducted elsewhere, using information from genome sequences to advance understanding of biology and improve health. The RSA Group has done extensive work to help the Sanger Institute build their leadership team, most recently helping them appoint their Head of DNA Pipelines and Senior Operational Manager.
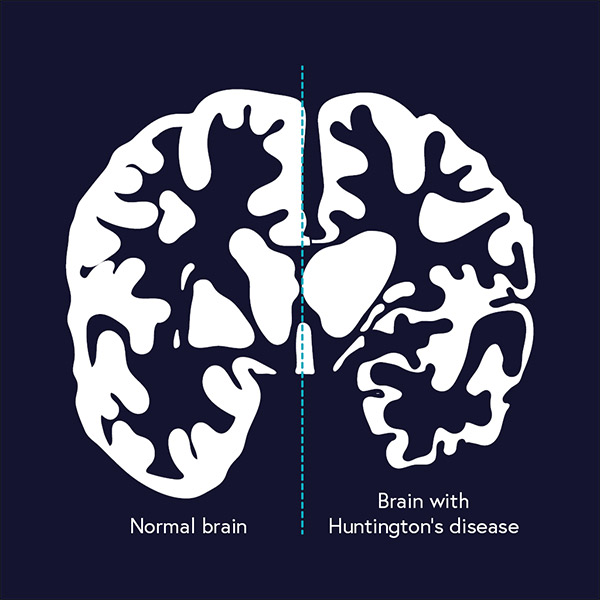
1993 – Huntington’s Disease gene is found
In 1993, a team led by geneticist Nancy Wexler identified the genetic mutation that causes the disease by working with a population in Lake Maracaibo, Venezuela. Pinpointing the gene opened opportunities for new research and for people who are at risk to take a simple genetic test to find out, with certainty, if they will develop Huntington’s disease or if they are in the clear. A cure for Huntington's, which usually strikes in midlife and is characterized by jerky movements, spasms, and eventual dementia, is not yet in hand, and could take years to develop.
1994 – The first gene linked to Alzheimer's disease is discovered
The association of Apolipoprotein E-4 (APOE) with the age of onset of common late-onset Alzheimer's disease (AD) was originally reported in three 1993 papers from the Duke ADRC (Alzheimer's Disease Research Center) group. The Center was investigating two diverse experimental streams that led to this discovery. After this relationship was first identified, more genes were found to be linked with the disease. Alzheimer’s disease is now considered a polygenic disorder. The APOE4 allele is still considered to be the strongest genetic risk factor for AD, and it is being thoroughly investigated ever since its discovery.
Our Inspiring Leader Baroness Susan Greenfield is CEO and Founder of NeuroBio, a privately-owned Biotech out of Oxford University with a therapeutic focus on neurodegenerative disease. The company has discovered a distinct mechanism and is developing exciting new drugs to treat Alzheimer’s disease. Baroness Greenfield is a neuroscientist and has published over 200 papers in peer-reviewed journals over the course of her 40-year career, based mainly at Oxford University. She holds 32 honorary degrees from UK and foreign universities, has received numerous honours including the Legion d’Honneur from the French Government, an Honorary Fellowship from the Royal College of Physicians, The American Academy of Achievement Golden Plate Award, and The Australian Medical Research Society Medal. She is also a Fellow of the Royal Society of Edinburgh. Our Executive Chairman, Nick Stephens, sits alongside her at NeuroBio as Chairman.
1995 - The National Institutes of Health announce the success of clinical trials testing the first preventive treatment for sickle cell anaemia
Hydroxyurea, also known as hydroxycarbamide, was the first approved drug for the treatment of sickle cell anaemia. It was shown to decrease the number and severity of attacks in 1995, and in a 2003 study it was shown to possibly increase survival time. This is achieved, in part, by reactivating foetal haemoglobin production in place of the haemoglobin S that causes sickle cell anaemia. Hydroxyurea had previously been used as a chemotherapy agent, and some concern exists that long-term use may be harmful, but this risk is either absent or very small and the benefits likely outweigh the risks.
1996 - The Cloning of Dolly the Sheep
This was one of the biggest stories spread through the public that science has ever seen, as “Dolly” the sheep was cloned in 1996. The project was led by Ian Wilmut of the Roslin Institute. As the first mammal to be cloned from an adult cell, with the same genetic identity, Dolly was a huge achievement, proving that the process of cloning found in nature could be attributed to organisms it does not naturally occur in.
Later in 2003, the first cloning of an endangered animal took place with the banteng, which was a great example of how cloning could help save species of animals that may not still be here in our lifetime.
1997 - KLOTHO aging gene discovered
Clotho was one of the mythical Greek fates who acts as a “spinner” of life. A disambiguation of this figure was a fitting title for a longevity-related genetic enzyme. Discovered by Japanese-born researcher Makoto Kuro-O and his colleagues in 1997, degenerations or mutations in this gene are linked to skin atrophy, osteoporosis, bone loss, and other signs of early aging. The klotho gene was originally identified as a putative age-suppressing gene in mice that extends life span when overexpressed. It induces complex phenotypes resembling human premature aging syndromes when disrupted. Since then, various functional aspects of the klotho gene have been investigated, leading to the identification of multiple novel endocrine axes that regulate various metabolic processes and an unexpected link between mineral metabolism and aging.
1998 - DuPont Gets FDA Clearance for Once-a-Day AIDS Drug, Sustiva
Efavirenz, brand name Sustiva, is an antiretroviral medicine used to slow down the progress of HIV infection. Although it is not a cure, the invention of this drug has bettered the lives of millions of people, especially at a time when the HIV epidemic was at its most rampant stage. HIV destroys cells in the body, called CD4 T cells. These cells are a type of white blood cell and are important because they are involved in protecting your body from infection. If left untreated, the HIV infection weakens the immune system so that your body cannot defend itself against bacteria, viruses and other germs. Efavirenz slows down the progress of HIV infection by reducing the amount of virus in your body, stopping the virus from replicating itself.
Sustiva was invented by DuPont Pharma a few years before 1998, when they turned to RSA to build their European launch team and the Regulatory team that made the registration of the drug possible.
1999 – GW pharma starts the first clinical trials with cannabis-based medicine
British Pharmaceutical company GW Pharma was founded in 1998 by Geoffrey Guy and Brian Whittle. The pair shared a common interest in plant medicine and were particularly intrigued by the potential efficacy of cannabis-based medicines for pain relief, as this field was vastly understudied in comparison to opioid derived pain medication. The journey of studying cannabis in relation to pain management was not an easy one, as trials were complicated to conduct and at first failed in their early stages. After more than 10 years of clinical development the first cannabis-based pain medication reached the UK market. In June 2010, the MHRA approved the medication Sativex as a licensed medicine to treat spasticity symptoms of multiple sclerosis patients. To this day Sativex is sold in 27 countries around the globe. However, GW’s journey into cannabinoid medicine did not stop with Sativex. In 2007 pre-clinical research into cannabidiol’s efficacy for treating epilepsy started and in 2013 clinical trials for Epidiolex began. By 2018 the drug was approved for use in the US, and one year later approved in the EU. To date over 15,000 people have used Epidiolex to treat epilepsy. Prior to GW’s formation Roger and Nick Stephens met and conversed with Geoffrey Guy and over time a professional relationship between the two parties flourished. This symbiotic relationship remains evident as collaboration continued up until GW’s acquisition by Jazz Pharmaceuticals in 2021. Most recently, the RSA helped appoint GW’s Head of Early Clinical Development, Kevin Craig.
2000 - Human genome mapped
In 2000, scientists from across the world finished a rough draft of the map of the human genome. The final version was realized in 2003. This biological breakthrough was a difficult accomplishment to reach. It took more than 10 years and contributions from hundreds of scientists.
The Human Genome Project reveals in intricate detail exactly what it is that makes us human, showing the placement of every chromosome that contains all the genetic material that makes us who we are. With the information from individual genome maps, scientists can not only discover genetic diseases better, but also uncover new clues about everything from a person’s body-odour to that person’s tendencies towards addiction.
2001 - The First Gene-Targeted Drug Therapy is Approved – Gleevec
An amazing development in the history of gene therapy and a great way to start out the 2000s, a well-conceived targeted gene therapy was approved by the U.S. FDA, and was sold as an anticancer drug to treat chronic myelogenous leukaemia (CML) by Novartis. This drug was called Gleevec (Imatinib), and is still used today as a cancer treatment drug. Oxford Biomedica, one of their major vector suppliers, is a pioneer of gene and cell therapy, with a leading industry position in lentiviral vector and cell therapy research, development, and bioprocessing. The company was one of the first in the UK biotech sector to go public, and The RSA Group has worked with them to build the majority of their Board, firstly by appointing ex Chair Lorenzo Tallarigio, among many other board and executive members, such as NED Heather Preston, COO Nick Page, CPO Helen Stephenson-Ellis, and CFO Stuart Paynter.
2002 – Synthetic Viruses and Biological Weapons
In 2002, researchers at the State University of New York created the world’s first synthetic virus. Using only an online instruction guide and chemicals ordered from a commercial company, the scientists made a synthetic replica of the polio-virus. The man-made disease was tested on mice and the symptoms observed were identical to that of the natural poliovirus i.e., paralysis and ultimately death. This study raised concerns over security, particularly due to the context of recent terror attacks in the United States. Anthrax, a chemical that is most known for its use as a biological weapon during the first world war, resurfaced in post-9/11 America. The renewed relevance of biological weaponry and warfare has encouraged researchers to seek potential antidotes. In the same year, University of Chicago oncologist Wei-Jen Tang’s research took us one step closer to developing the treatments available today.
2003: The FDA approves Palivizumab (trade name Synagis), to prevent serious lower respiratory tract (lung) disease caused by respiratory syncytial virus (RSV) that would require hospitalisation
After spending more than a decade at NIH and Johns Hopkins University, Greg Prince co-founded Virion Systems, Inc. (VSI), a biotechnology company focused on the prevention and treatment of paediatric infectious diseases. Building on discoveries made by Prince as a doctoral student, VSI pioneered the prevention of respiratory syncytial virus (RSV) disease in high-risk infants, the primary cause of infant pneumonia throughout the world, by using monoclonal antibodies to prevent RSV. VSI's technologies were licensed to MedImmune, and their collaborative efforts resulted in FDA approval of Synagis, a drug that is currently used to treat approximately a quarter of a million high-risk infants throughout the world each year.
Our Executive Chairman Nick Stephens and Greg Prince maintain a close relationship, as Nick has the pleasure to sit on the board of Soft Cell Biological Research, where Greg acts as President of the company.
2004: John Van Tam begins his career in Public Health
Over our 40 years, RSA has shaped the careers of several noteworthy members of the life science community. One of the more significant of these figures is Professor Jonathan Stafford Nguyen Van Tam. John Van Tam was placed at Roche by RSA in 2001 as Head of Medical Affairs, however his seminal work in the UK government began in 2004 when he took on the role of consultant epidemiologist and Head of the Pandemic Influenza Agency. In the same year, his expertise in infectious diseases led him to consult the World Health Organisation on influenza, cementing his position as one of the world’s leading scientists in the space. Van Tam’s influence on public health policy continued to spread as he was appointed to the Scientific Advisory Board for Emergencies during the Swine Flu Pandemic of 2009/10. Today, Van Tam is the deputy CMO for the Ministry of Health and as a result has been a key figure in forming the UK government’s response to the COVID-19 pandemic.
2005 – Chris Whitty appointed to London School of Hygiene and Tropical Medicine
In 2005 the RSA group helped shape the career of yet another prestigious and world-renowned medic Chris Whitty. He was placed in the London School of Hygiene and Tropical Medicine where he began research into infectious diseases in the UK, Africa and Asia. Subsequently, he was appointed as the Director of the Malaria Centre, and in 2008 the institute received a £31 million grant to study Malaria in Africa by the Bill and Melinda Gates foundation. Dr Whitty currently works the Chief Scientific Advisor and Chief Medical Officer in the UK’s Ministry for Health, and consequently, has been responsible for the enormous task of leading the government’s response to the COVID-19 pandemic.
2006 - FDA Approval of the First Preventative Cancer Vaccine
A joint venture - Sanofi Pasteur MSD, owned on a 50/50 basis by Sanofi Pasteur and MSD, was created in 1994 to develop and commercialize vaccines originating from both companies' pipelines to improve and promote public health in 19 European countries. In 2006, Sanofi Pasteur MSD launched a ground breaking cervical cancer vaccine called Gardasil. This was the first preventative cancer vaccine to ever reach the market. At this time, it was approved only for females aged 9-26, however by 2009 it was also approved for males of 9-26 years. The popularity of the inoculation was substantial because it is a recombinant vaccine. By 2018, Gardasil was approved for males and females aged 9-45, showing its effectiveness during the past decade both in the market and through continued academic study. To date, this HPV vaccine is still the only preventative cancer option, though the Hepatitis B vaccine is known to reduce the risk of liver cancer.
2007 - Stem cells created from mature skin cells
In 2007, two separate teams of scientists from Kyoto University and the University of Wisconsin-Madison reverted adult skin cells to simulate the actions of pluripotent stem cells. Pluripotent stem cells can differentiate into nearly all cells and were previously only found in embryonic stem cells. This new process of creating induced pluripotent stem cells from mature cells changed the “programming” that makes them become skin in favour of acting like embryonic stem cells that could end up being virtually any kind of cell.
Embryonic stem cells were one of the most promising areas of medical research with the potential to cure diseases from diabetes to cancer to genetic disorders, but ethical concerns had largely curtailed their use. This discovery allows such research to continue without the ethical concerns or legal restrictions attached to embryonic cells. Also, it gives biologists the possibility to grow replacement organs for people using cells with their own DNA, reducing the probability of organ rejection.
Lord Digby Jones, UK Minister for Trade and Investment, presented the Award to Nick. The Minister said that it recognised RSA’s achievements in competing in the global marketplace. His Dissertation was entitled: ‘Development of a Practical Approach to Improve Compliance with Anti-depressant Therapy’. His internship placement was at Pope Woodhead
Foster Partners and RSA combined, enjoy over 50 years of experience in specialist Executive Search and Interim Management. (February08-37a) (February08-44a) (February08-45a)
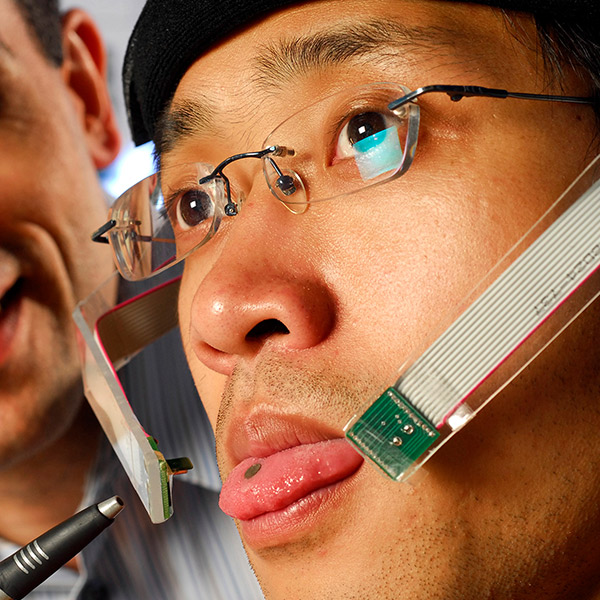
2008 – Tongue Drive system shows potential for debilitating disabilities
Researchers developed an experimental tongue-based system that may allow individuals with debilitating disabilities to control wheelchairs, computers and other devices with relative ease. Because the tongue is directly connected to the brain via cranial nerves, it usually remains mobile when other body parts lose function due to disease or accidents. That mobility underlies the new system, which may one day provide greater flexibility and simplicity to individuals who would otherwise use sip-and-puff controls or brain implants. Electrical engineer Maysam Ghovanloo developed the Tongue Drive system in collaboration with graduate student Xueliang Huo and presented the findings on June 29 at the 2008 Rehabilitation Engineering and Assistive Technology Society of North America (RESNA) Annual Conference in Washington, D.C.
2009 - First time ever that two women have shared credit for one of those Nobel prizes in Chemistry, physiology or medicine
The prizes in chemistry and in physiology or medicine were both divided into three parts in 2009. And of those six total prizes given, half were given to women [Ada Yonath of Israel in Chemistry, and Elizabeth Blackburn and Carol Greider of the U.S. in Physiology or Medicine]. This was the first time ever that two women had shared credit for one of those Nobel prizes.
There are plenty of women who have made important contributions to science: Marie Sklodowska Curie, Rosalind Franklin, Mae Jemison, Catherine Johnson, and Rachel Carson, to name a few. As society begins to accept science as a space in which men and women alike can operate, old biases might eventually fall away. And as more women start seeing STEM as a viable and acceptable career option, more women will pursue work in the field but there is still a lot of work to do to keep them there. It continues to require not only a change of perception but also real adjustments and effort on the part of employers and social policy makers.
2010 - HIV prophylaxis
In 2008 the UN reported 2.7 million HIV infections occurred worldwide. The HIV virus is known to disproportionately affect men that have sex with men. To address this public health issue in 2010 the Bill and Melinda Gates Foundation funded a study that was conducted on homosexual men to determine the efficacy of a potential preventative HIV medicine. During study, subjects were administered emtrialabine (FTC) and tenofovir disoproxil fumarale (TDF) which was combined into one pill known as FTC-TDF. The study findings indicated for the first time the effectiveness of the FTC-TDF combination as a viable prophylactic method for HIV. Pre-exposure prophylaxis is now standard practice, and advancements in the field include exploring different modalities of administration to move away from daily intake to a monthly intake through subcutaneous devices.
2011 - Greater understanding of relationship between cell replication and ageing
Prior to 2011 scientists had theorised and debated whether a link existed between the symptoms of ageing experienced by a given organism and the cells residing within it. In November 2011, a study conducted by Baker et al at the Minnesota Medical School demonstrated evidence of a relation between cells that have reached their replication limit (labelled senescent cells) and the increased malfunctioning of bodily processes that manifest as the symptoms of ageing. This novel mechanism of understanding the ageing process has implications for a wide range of diseases from cancer to dementia, as well as general cosmetics.
2012 - Discovery of CRISPR Genome Engineering Tool
In 2012, Jennifer Doudna, Emmanuelle Charpentier, and their teams elucidated the biochemical mechanism of CRISPR technology. By making precise targeted cuts in DNA, CRISPR ushered in endless potential in areas of medicine, agriculture, biomaterials, etc.
CRISPR-Cas9 is a bacterial adaptive immune system in nature, whereby pieces of DNA from invading viruses are snipped off by a bacteria nuclease CRISPR associated protein. The DNA fragment that is chopped off is saved as memory for fighting future infections. The CRISPR-Cas9 system can be engineered to edit eukaryotic DNA by designing guide RNA complementary to the target sequence. What CRISPR technology is being used for varies from developing cancer treatments, to tackling obesity, to creating hornless cows - and so much more. Whether it is diagnostics, treatment, or something else, there are new discoveries every day coming to the forefront of this field. Because of this outstanding achievement, Jennifer Anne Doudna received the 2020 Nobel Prize in Chemistry, with Emmanuelle Charpentier, making them the first ever all-female recipients of the Chemistry Prize.
2013 - Developing understanding of human microbiome
The human microbiome refers to genes contained within microbial cells found in every living organism, including humans. Since the term was coined in 2001 researchers have devoted much time to narrowing this definition and understanding its potential within pharmaceutical medicine. In 2013, a study from the University of Colorado (Boulder) revealed four major implications about the human microbiome. Firstly, it was evidenced that microbiome genes change in response to significant environmental factors, for example when an infant change from breastmilk to solid foods. Additionally, although the diversity of the microbiome varies wildly within individual human hosts, the most stable and consistent environment is the gut. It was also suggested that the microbiome within the gut plays a significant role in digestion, with data indicating that microbes in our food could provide human microbiomes with new genes that aid digestion. Finally, and perhaps most significantly, despite the stability of the human gut microbiome over time, studies found evidence of plasticity after exposure to antibiotics in selected individuals. This finding may have profound implications in the field of personalised medicine, as analysing an individual’s microbiome could determine the most effective treatment pathway.
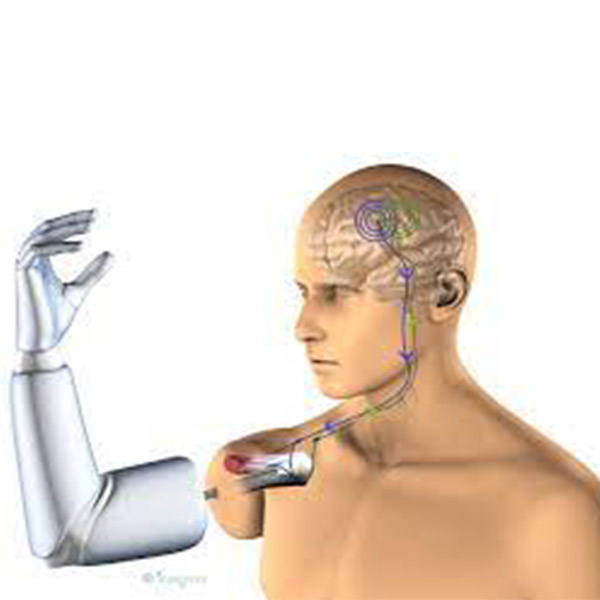
2014 - Robotic limbs fully controlled by the brain
In 2014, the U.S. Food and Drug Administration approved the first prosthesis controlled by neural signals from the wearer’s brain for use by the general public. This is the culmination of almost two decades of biomedical research. In 2000, researchers at Duke University Medical Center implanted electrodes in monkeys’ brains to control a robotic arm and pick up food. By 2004, a noninvasive method of picking up brainwaves was developed and being used to control biomedical devices. The RSA Group is currently collaborating with the NTU School of Medicine in Singapore to appoint a new faculty member for their Rehabilitation Medicine Department, working together to achieve their goal of becoming leaders in the field of Rehabilitation Medicine.
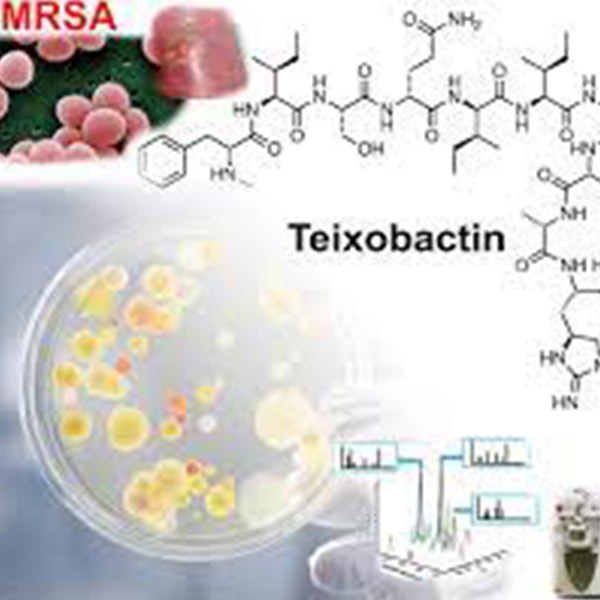
2015 – Discovery of Teixobactin
It is predicted that in the future antimicrobial resistance (AMR) will be responsible for more deaths than cancer. This has led to research being focussed on producing new classes of antibiotics with novel modes of action to combat the rampant spread of resistance encumbering classic therapeutic agents. One such recently discovered molecule is teixobactin, which has shown excellent activity against a range of clinically relevant, Gram-positive pathogens including methicillin-resistant Staphylococcus aureus (MRSA), Mycobacterium tuberculosis, and Enterococcus spp. (vancomycin-resistant enterococci, VRE). Teixobactin is a recently described antibiotic of a new class produced by a hitherto undescribed soil microorganism (provisionally named Eleftheria terrae). The compound was identified using a new method to grow uncultured organisms by cultivation in situ, and was found to inhibit cell wall synthesis by binding to a highly conserved precursor.
2016 - MiNA Therapeutics kicks off first-ever saRNA nanoparticle trial
Developer of therapeutic medicines designed to harness gene activation mechanisms through small activating RNA (saRNA), MiNA Therapeutics first developed their platform in 2016. Through transcriptional activation, saRNA therapeutics promise a revolution in our ability to modulate previously undruggable targets. The company's technology and clinical know-how help transform the therapy landscape of cancer and other severe diseases, enabling doctors and physicians to cure diseases and develop medicines that restore normal function to patients' cells. The RSA Group collaborated with MiNA Therapeutics in their expansion phase where we helped them appoint a VP of Clinical Operations, enabling them to grow into the world leading company they now are.
2017- First CAR-T Therapy for Cancer is Approved
At the National University of Singapore Prof. Dario Campana began pioneering work in the oncology space that would later become known as CAR T Immunotherapy. In 2017, two CAR T-cell therapies were approved: one for acute lymphoblastic leukaemia in children, and the other for advanced lymphoma in adults. This is a huge step for CAR T therapy, which if treatments prove as effective as expected, will likely replace chemotherapy as the primary form of cancer treatment. This will see a huge benefit to patients’ experience as CAR T is non-toxic to the human body and has demonstrated complete ablation of cancerous tumours in as little as 10 days. By combining T cells with Chimeric Antigen Receptors (CAR) patients’ immune cells are reprogrammed to attack cancer cells without damaging others. Following this success, Prof. Campana went on to co-found Medisix Therapetuics, a biotech company that produces CAR T
2018 - Evolving Techniques for Tracking Single Cell Development
Over several years geneticists have worked on combination of techniques to detect which genes within cells are “active”. The method has been labelled single cell RNA-seq, and allows scientists to isolate the specialised roles of individual cells, known as transcription factors, by examining which proteins activate specific biological functions. Resultantly, this allows an organism’s development to be tracked cell by cell over time. Scientist within related fields believe that this novel technique will revolutionise the way genetics are researched and will permit a more nuanced understanding of how cells develop, regenerate and the way they are manipulated by disease. In 2018, after years of intense study, the technology had been developed to the point at which it was capable of tracking early cell development and was subsequently hailed as the scientific breakthrough of the year. The implications of this breakthrough extend into many areas of biology including epidemiology, embryology, and gerontology.
2019 - Changing the course of disease (Director for Africa Centre)
In 2012, The RSA Group placed Marie-Louise Newell as the Director of the Africa Centre for Health and Population Studies. Her expertise in the field of epidemiology went on to contribute to the battle against the 21st century’s most deadly diseases.
In response to the 2014-2016 Ebola outbreak in West Africa, public health officials and the pharmaceutical company Merck fast-tracked rVSV-ZEBOV, an experimental Ebola vaccine. After a highly successful field trial in 2015, European officials approved the vaccine in 2019—a milestone in the fight against the deadly disease. Several landmark studies also opened new avenues to preventing the spread of HIV. A 2011 trial showed that preventatively taking antiretroviral drugs greatly reduced the spread of HIV among heterosexual couples, a finding confirmed in follow-up studies that included same-sex couples.
2020 - COVID-19 Testing Programme Launches
In March 2020, the UK government announced that it would be launching a national testing effort to support the UK’s fight against coronavirus via the establishment of a network of “mega” testing laboratories, known as “Lighthouse Labs”. In 2017 the RSA placed Professor Peter Simpson as the Chief Scientific Officer for Medicines Discovery Catapult, an acclaimed CRO that collaborates with pharma and biotech companies to discover new drugs. Medicines Discovery Catapult was tasked with the challenge of co-ordinating the creation of the UK’s Lighthouse Labs Network and delivery of one of those labs, based at Alderley Park in Cheshire. Professor Chris Molloy, Medicines Discovery Catapult CEO, and former CEO of The RSA Group, was appointed Director of the UK Lighthouse Labs Network, whilst the CSO Professor Peter Simpson was appointed Director of the Alderley Park Laboratory. The Lighthouse Labs take their name from the PCR testing technology which uses fluorescent light to detect the virus. With an upstanding spirit of collaboration for the greater good, Universities, research institutes and commercial companies across the UK lent testing equipment and expertise in response to the vital national need.
2021 - Companies Develop Covid-19 Vaccines in Record Time
Covid-19 dominated science coverage in 2020-21, and rightly so. The world grappled with how to combat the SARS-CoV-2 virus, learning about how it spread and how it affected the human body.
While the disease continues to spread around the world and cause death, help arrived thanks to the record-setting effort to develop vaccines. In less than a year, Moderna and Pfizer, in cooperation with BioNTech, created the first messenger RNA (mRNA) vaccines to protect against Covid-19. This vaccine contains a synthetic version of RNA that tricks the immune system into thinking a virus is present and therefore produces antibodies designed to combat it. This differs from traditional vaccines, which are made of small amounts of an existing virus.
In 2012-2013, the then CSO of Moderna, current CEO of Evox Therapeutics Ltd, and one of our featured Inspiring Leaders, Antonin DeFougerolles proceeded to test vaccine constructs. He presented the concept of mRNA technology as a rapid response platform for pandemic and biodefense purposes to the National Institute of Allergy and Infectious Diseases (NIAID). In 2013, they received their first US government grant to begin funding work in the area. Since then and over the next half dozen years the team at Moderna and other mRNA companies have paved the way by further optimising the mRNA platform through iterative testing in clinical trials. This work has resulted in the development of over 25 different mRNA vaccine drug candidates for various pathogens and cancers. Read more from Tony about the early foundations of Moderna's mRNA technology and the back story to its now flourishing use in vaccines in this LinkedIn article.
Adrain Cottrell, CIO at The Institute of Cancer, London
Now appointed CIO at The Institute of Cancer Research in London, one of the leading Comprehensive Cancer Centres in the world with the primary goal of defeating cancer, prior to that Dr Cottrell held the role of Vice President in GSK for ~10 years focused on transformation of R&D through the use of technology.
During a 30 years career at GSK he held a number of different global roles and led activities through several significant mergers, acquisitions and divestments, reflecting his passion for driving business efficiency and compliance through the deployment of simple Tech solutions. Recent successes have seen the introduction of Veeva, Robotic Process Automation (RPA), High Performance Computing (HPC) and Data Visualisation solutions.
He is also a board member of the Accenture Life Science Consortium, which aims to bring industry standard solutions to meet non-competitive business challenges. Partners include E&Y, to establish an industry response to the EU regulations for 'IDMP', and SAS, to create a cloud-based solution to enable the sharing of anonymised patient data to promoted great transparency of big Pharma.
“Successful delivery is achieved by creating teams with a culture that combines a drive to achieve the goal with respect for the contribution of each team member. Strong governance and clear communication underpin everything”.
William Burns, Company Director at Shire, Mesoblast, Vestergaard, Molecular Partners, Wellcome Trust; Institute of Cancer Research London
Paving a career that has uniquely helped shape the Life Science field, Mr. Burns joined the team from Roche Pharmaceuticals, where he worked for 28 years culminating in his tenure as CEO from 2001 to 2009. His board contributions during that time included seats at Roche, Genentech and Chugai Pharmaceutical. Mr. Burns was also non-executive director and chairman of BioTie Therapies Corp. Since 2010, he has been non-executive director and senior independent director of Shire Pharmaceuticals. More recently, Mr. Burns has been chairman of Vestergaard S.A. as well as Molecular Partners, and vice-chairman of Mesoblast.
Mr. Burns is also a trustee and governor of the Wellcome Trust Ltd. and a trustee of the Institute of Cancer Research, London. He is a member of the Novo Holdings Advisory Group and a member of the Scientific Advisory Board of the Center for Integrated Oncology of the University of Cologne/Bonn. Mr. Burns holds a bachelor’s degree in economics from the University of Strathclyde, Glasgow.
Bruno Febra, Head of Commercial and Business Development Generics EU/ROW, Management team at Alter Pharma Group
With specialty experience on all the steps concerning the development, manufacturing and commercializing a generic medicine, Bruno Febra’s versatile commercialization knowledge encompasses many markets included work has spanned across continents focussing on commercialvaries markets including Europe, USA, Asia, and LATAM.
Previously holding business roles at Bluepharma and Pharmathen, he is now Head of Commercial and Business Development Generics EU/ROW at Alter Pharma Group.
Christian Joergensen, International CEO, Chairman and non-exec board member
"It is rewarding to work within the lifescience industry not only because of the obvious satisfaction of being able to improve the life of so many people, but also because of the international nature of the area and the diversity of people/skills necessary to succeed. Having worked in 5 different countries has given me a great network of highly talented people and knowledge of how to use diversity to improve results."
Dr Colton Wong, Senior Director at Wuxi AppTec China
Senior Director of Wuxi AppTec, Dr Wong reshaped the company’s bioanalytical capabilities with his extensive experience in the bioanalytical industry and strong leadership skills, heading the Shanghai Small Molecule Bioanalysis Method Development team.
As an esteemed market leader and speaker, Dr Wong frequently participates in industry-leading discussion panels and conferences and has been recognised his talents through the win of the LTD Science Day Competition 2019, and the Young Scientist Award in the Chinese Bioanalytical Forum.
David Arias Gayo, General Manager Mibe Pharma Spain and Allergopharma Spain
Throughout his 25 years’ career, Mr Arias Gayo has built incredible competence covering different and varied commercial, marketing and communication roles in the pharmaceutical industry at an international level, earning him exceptional expertise about the field.
He now manages Allergopharma Spain, one of the leading companies in the field of Allergen Immunotherapy (AIT), and Mibe Spain, owned by Dermapharm AG.
Dr. Dieter Braun, Head Specialty Brands and Hospital Business at Accord Healthcare
A highly experience global biopharmaceutical commercial executive, Dr. Dieter Braun has been a remarkable driver of change and business development in every company he has held a position in. Heavily skilled and experienced in all aspects surrounding product launches and commercialization, he has had a string focus on specialty products and biosimilar, implementing strategies for Neovii Biotech, CINFA Biotech, and Mundipharma International.
Dr Braun is now working for Accord Healthcare, sharing and implementing their vision of making widening access to pharmaceuticals therapies more accessible worldwide by developing, manufacturing and distributing affordable medicines.
Elmar Schnee, Chairman and Board Member
An industry shaping influence, Mr Elmar Schnee chairs a plethora of companies, acting member of the board for Moleac, as Chairman for Calliditas, Santhera, Genkyoyex, Jazz Pharmaceuticals, ProCom RX, and as NED for Advanz Pharma. Previously acting as Advisor to Management of MindMaze, a neuro-technology company spun off from the Swiss Federal Institute of Technology in Lausanne (EPFL). Prior to that, he was Chairman, CEO and board member of Cardiorentis in Zug, Switzerland. Previously, he was a General Partner and member of the Executive Board of Merck KGaA, responsible for its worldwide pharmaceutical business. He also led the major restructuring of the business including the acquisition and integration of Serono. Prior to Merck, Elmar Schnee held senior roles in marketing, licensing, strategy, business development, and as Managing Director with UCB Pharma, Sanofi-Synthelabo, Migliara Kaplan and Fisons. At Santhera, in addition to his role as Chairman of the Board, Elmar Schnee is also a Member of the Compensation Committee.
Dr Fabio Magrini, Principal Medical Director USMA Neuroscience at Genentech.
Throughout the course of his 20-years of experience in the industry working for Pfizer, Amgen, Roche, MedImmune and Eli Lilly and Co, Dr Magrini has been consistently passionate about bringing clinical innovation, high quality standards and courageous leadership to all phases of drug development from translational medicine, PhI-III, and medical affairs.
He has initiated, designed, led, supported and implemented a large number of clinical programs in chronic inflammatory and autoimmune disease across several therapeutic areas including Neurology, Rheumatology, Nephrology, and Dermatology.
Over the last few years he has developed an interest in integrative medicine approaches and patient focused outcomes, particularly in regard to pharmaceutical development and clinical research.
He is now Principal Medical Director USMA Neuroscience at Genentech.
Dr Francois Martelet, NED Novigenix SA, CEO Osamia Pharmaceutical AB
Dr. Francois R. Martelet, MD MBA, is a Senior Advisor at Ennovance Capital. He is currently the Chief Executive Officer of TopoTarget, which is a biopharmaceutical company in Copenhagen that is engaged in the research and development of cancer treatments. Previously, he was President & CEO of Avax Technologies Inc., a US-based biotechnology company that specialises in the development of autologous cell vaccine for melanoma cancer patients.
Dr. Martelet worked for Merck & Co (Revenue over $23 billion/yr.) as a Vice President & Global Franchise Head and the Senior Vice President & Region Pharma Head with Novartis AG (Revenue over $42 billion/yr.). In this role, he had direct P&L responsibility (over $1 billion business) and led a team of 3,000 people within Central Europe, the Middle East, and Africa. He previously worked as Vice President & InterContinental Region Head of Oncology at Novartis. Under his leadership, these regions were among Novartis’ fastest growing, consistently exceeding both near- and medium-term profitable sales growth objectives.
Dr. Martelet received a Doctorate in Medicine and a Master's Degree in Business from Dijon University in France. He also holds a Degree in Legal Medicine from R. Descartes University School of Medicine in Paris, France. In addition, he attended the Advanced General Management Program at INSEAD in France, as well as several Senior Executive Programs at Harvard Business School.
Gareth Waldron, Director, Development Non-Clinical Safety at Sosei Heptares
Gareth brings 17 years industry experience, the vast majority with Pfizer, Neusentis, and Mission.
After graduating in Physiology and Pharmacology he successfully completed a PhD programme investigating the influence of endothelium-derived changes in smooth muscle membrane potential on vascular tone. He subsequently pursued 2 post-doctoral fellowships utilising electrophysiological and molecular biology techniques. Following his academic research, he decided to use his scientific skillset in a more applied environment.
He is an accomplished safety pharmacologist with a successful track record of leading both internal safety pharmacology teams and working with CROs to ensure the appropriate preclinical studies are conducted to enable successful progression of programmes to first in human studies.
Gareth now works as Director of Development Non-Clinical Safety at Sosei Heptares.
Giacomo Mordenti, Head of Biostatistics & Data Management Europe at Daiichi Sankyo Europe GmbH
An established executive leader in the Biostatistics / Data Management / Data Analytics space across multiple therapeutic areas along the drug and device development value chain, Giacomo brings over 20 years of experience from the pharmaceutical and medical device sectors.
Most recently, he was the Senior Director and Global Head of Statistics & Data Management at LivaNova. Before this Giacomo was the Senior Director and Head of Biostatistics at the Grünenthal Group and has also held senior roles at Merck Serono and Menarini Ricerche S.p.A.
Now working as Executive Director, Head of Biostatistics & Data Management Europe for Daiichi Sankyo, a global pharmaceutical company primarily focused on the development of novel therapies in oncology, rare diseases and immune disorders.
Dr. Glen Clack, Chief Medical Officer at Evgen Pharma PLC.
Glen has extensive experience in translational medicine, particularly the early development of anti-cancer compounds, from lead optimisation to clinical proof of concept in both solid and haematological malignancies. One of his many significant roles included Senior Medical Director in the Oncology Innovative Medicine Unit of Astra Zeneca. Since 2016, Glen has been involved with a number of innovative early-stage biotech companies in the oncology area.
Glen has been the industry representative for several academic and regulatory alliances and think tanks, was elected a Fellow of the Faculty of Pharmaceutical Medicine in 2012, sits on the Science and Innovation Advisory Committee of the UK Bio Industry Association, and is a tutor and lecturer in the School of Pharmacy at Manchester University. Glen is honorary professor of translational medicine at the University of Sheffield, where he completed his medical degree.
Graham Price, Head Translational Medicine, UCB New Medicines at UCB
Graham Price has 15 years of experience in clinical development. He is currently Director of Clinical Pharmacology in Global Early Development at UCB, working in the areas of Immunology and Neurology. Previously, he was Chief medical Officer at Oxford BioMedica Ltd, responsible for all medical and clinical research activities leading the clinical development group and accountable for global product development.
Prior to this position he was Vice President, Clinical Development for MedImmune Ltd in Cambridge UK, where he was accountable for product development in Europe and Vice President, Clinical Development at Takeda Global R&D where he was Global Clinical Lead for cardiovascular and metabolic products.
He is a Fellow of the Royal Society of Medicine as well as the Faculty of Pharmaceutical Medicine at Imperial College, and has published on various topics including Alzheimer’s disease, hypertension, dyslipidaemia, ocular gene therapy and treatment of cervical malignancy.
Dr Heather Preston, Managing Partner at Pivotal BioVentures
Heather Preston MD joined Pivotal in 2018 and brings 30 years of healthcare experience. Most recently she was a Firm Partner and Managing Director at TPG Biotech and is currently a Senior Advisor to TPG. Prior to joining TPG in 2005, Dr. Preston spent two years investing with JP Morgan Partners and she was also an Entrepreneur-in-Residence with New Enterprise Associates. Previously, Heather spent five years at McKinsey & Co. in New York, where she lead their pharmaceutical and medical products consulting practice. She advised pharmaceutical companies and biotechnology companies on strategic issues such as R&D portfolio prioritization, M&A opportunities, new technology acquisitions, new product launches and product growth strategies.
Heather currently serves on the board of directors of Oxford Biomedica ; Karuna Therapeutics ; Entasis Therapeutics, Unchained Labs, Azura Opthalmics, Fusion Pharmaceuticals, Akouous and Avalyn Pharmaceuticals. She has previously served on multiple private and public boards including Albireo, Alder BioPharmaceuticals (Acquired by Lundbeck in 2019), Aptalis Pharma (acquired by Forest Labs in 2014), and Elevation Pharmaceuticals (acquired by Sunovion in 2012). She was responsible for TPG Biotech’s investment in Par Pharmaceuticals (acquired by Endo Pharmaceuticals in 2015).
During her academic medical career, she was the recipient of a Fulbright Scholarship, a Fulbright Cancer Research Scholarship, a Harlech Scholarship and a Science and Engineering Research Council Post-doctoral Fellowship Award. Dr. Preston also serves on the Board of Trustees for the Harvard Discovery Council, the Fine Arts Museums of San Francisco, Saint Luke’s School and the Harlech Scholarship.
Dr Huw Jones, Chief Executive Officer at Evgen Pharma PLC
For over 20 years, Huw Jones has been a serial CEO and NED of early stage and listed biotech companies. Throughout his varied career he has launched dozens of new medicines in several countries, completed tens of M&A transactions and managed many drug development programmes.
In October 2020 he was appointed CEO of Evgen Pharma PLC, an AIM listed clinical stage cancer and inflammation company. He recently led the raising of £10m in a placing and up to a further £1m in an open offer in a heavily oversubscribed and transformative deal for Evgen. Board roles include NED of Ixaka Ltd (formerly Rexgenero), a cell and gene therapy company with operations in London, Paris, and Seville; and NEC of Chronos Therapeutics Ltd in Oxford. Huw is also a strategic advisor to Cambridge based Gen2 Neuroscience, an antibody company focussed on tauopathies such as Alzheimer’s Disease. He was surprised and delighted to have been awarded European CEO of the year 2016/17 for drug discovery by European CEO Magazine, alongside leaders in their sectors from Adidas, Unilever, and Spotify.
Ian Crosbie, Chief Executive Officer at Sequana Medical NV
With 25 years of experience, both in-house at medical device and pharmaceutical companies, and as an investment banker at several leading global firms.
Prior to joining Sequana Medical, Mr. Crosbie was Chief Financial Officer of GC Aesthetics Ltd. based in Dublin. Before that, Mr. Crosbie was Senior Vice President, Corporate Development at Circassia Pharmaceuticals plc, a late-stage biopharmaceutical company focused on allergy immunotherapy where he led the execution of the company's £210 million IPO, as well as the M&A & licensing activities. Prior to Circassia, Mr. Crosbie enjoyed a 20-year career in corporate finance including positions such as Managing Director, Healthcare Investment Banking at Jefferies International Limited, as well as Director, Healthcare Investment Banking at Deutsche Bank. In addition, he was a founding partner at Gargoyle Partners / Ferghana Partners.
Mr. Crosbie served as a Second Lieutenant in the 32nd Armoured Engineer Regiment of the British Army and earned a degree in Engineering, Economics and Management from Oxford University.
James Culley, Director at JRC Consultancy
James has a wide range of Director, Business Management, Key Account Management and Commercial acumen which is comprised of over twenty-five years direct sales experience.
Over the past 10 years, James also developed a successful commercial property portfolio.
Before serving the role of Business Development Manager at Mosaic Pharma, his previous position was with Wockhardt ltd as UK and Ireland Head of Business Development, with responsibility for horizon scanning and the entire Hospital and OTC product pipeline development.
During his time at Ratiopharm, he specialised in new Bio-similar, Oncology, Hematology injectable and oral solid dose Generic pharmaceutical product launches.
James is now Director of JRC Consultancy, where he acts as a consultant to the Pharmaceutical and Medical Industry.
John Overington, CIO, Medicines Discovery Catapult
In 2017 John joined the Medicines Discovery Catapult as CIO where he leads the development and application of informatics approaches to promote and support innovative, fast-to-patient drug discovery in the UK through collaborative projects across the applied R&D community.
John joined from technology company BenevolentAI, where he was involved in the development of novel data extraction and integration strategies, integrating deep learning and other Artificial Intelligence approaches to drug target validation and drug optimisation.
Prior to this, John worked for Inpharmatica, where he led the development of a series of computational and data platforms to improve drug discovery, including the medicinal chemistry database StARLite. In 2008 he was central to the transfer of this database to the EMBL-EBI, where the successor is now known as the ChEMBL. More recently, the work extended into large-scale patent informatics with the Open patent database SureChEMBL. John is a visiting professor at UCL and the University of Manchester.
Nick Page, COO at Oxford BioMedica
Nick Page has served as Chief Operating Officer of Oxford Biomedica since April 2019. Prior to this he has held a number of senior operational leadership positions in the pharmaceutical industry, most recently as Platform Head of Anti-infectives within Novartis. His 40+ years of industry experience include API, Solid oral dose, Sterile, and Radiopharmaceutical manufacturing in various organisations varying across the innovative, generic and contract manufacturing sectors. During his career he has spent several years working in China and India as well as in Global roles.
Nigel Brooksby, Chairman and Non-Executive Director at Neurocentrx Pharma Limited
Nigel Brooksby is highly regarded as an experienced Businessman and Entrepreneur in the Life Sciences arena. Over the last seven years, Nigel has been involved in raising significant investments. In 2015, he was instrumental in gaining a listing for a clinical stage Biopharma company on NASDAQ and continues to be a key investor and director. He has been a long-standing investor in a novel biotechnology company that gained an AIMS listing in early 2017. Nigel has undertaken many projects in partnering biotech companies with big pharma. He is also a Non-executive Director of Porton Biopharma and Chronos Therapeutics, a Director of Stabilitech Biopharma, and Chariman of Neurocentrx Pharma Ltd.
Previously he held senior Global Appointments in Wellcome (now GSK), Pfizer and until his recent retirement was a Member of the Sanofi Executive Committee in Europe and North American and Chairman and Managing Director of the Sanofi Group in the UK and Ireland. He has worked internationally in Europe, the USA, Latin America, the Middle East and Africa.
Nigel is a past President of the Association of the British Pharmaceutical Industry (ABPI) and received Honorary Lifetime Member status in recognition of his services to the Association and the Life science Industry.
Nigel holds several Academic, Charity and Life Sciences Chairmanships and Senior Board positions, which include the Clinical Trials Unit at Imperial College London, advising the Wellcome Trust and the South Australian Health Board, a Visiting Lecturer of the Singapore Management University and a Trustee of the Loulou Foundation.
Professor Peter Simpson, CSO at Medicines Discovery Catapult.
Peter is currently the CSO at Medicines Discovery Catapult, where he helps drug discovery companies through collaborative research and bioanalytical innovation, and develops strategic partnerships to enable access to state-of-the-art technologies for UK innovators. Prior to this Peter has most recently been the Director of N8 Research Partnership, the not-for-profit body that establishes multi-university research collaborations across the North of England. Peter led strategic engagement on innovation policy in the Northern Powerhouse with government ministers and departments, and with Local Enterprise partnerships, and built a strong evidence base for Northern industrial research strengths.
With extensive experience in drug discovery in major pharmaceutical companies, Peter brings a wealth of open innovation expertise and strategic early discovery knowledge. Peter worked for AstraZeneca in Alderley Park for nine years. There, Peter directed assay development, high throughput and safety screening, and sample management departments, and led oncology discovery projects. He pioneered AstraZeneca’s industry-leading Open Innovation strategy for early discovery. Peter was responsible for setting up successful discovery partnerships with international organisations including: European Lead Factory, MRC-T (now LifeArc), Lead Discovery Centre Dortmund, NeoMed in Canada, Bayer Pharmaceuticals, and Academic Drug Discovery Consortium in the US. Prior to that, Peter worked in Neuroscience project and cell imaging management roles for Merck Sharp & Dohme.
In 2019 Peter was appointed Honorary Professor at The University of Manchester.
Pierre van Weperen, CEO at Grow Pharma, Managing Director at Grow Group UK and Chair of the Board at Dutch National MS Society
Pierre has over 19 years of leadership experience and a track record of leading, running and revitalising small-medium businesses both in the pharmaceutical industry and in services at Novo Nordisk, MSD and Wyeth. Notably, he led Novo Nordisk’s UK Diabetes team, as well as being the Executive Director for Primary Care (General Medicine) at MSD.
Previously he was at Ashfield, part of UDG Healthcare, a leading international healthcare services provider, where he held the role of Commercial and Patient Solutions UK Managing Director.
He is now CEO at Grow Pharma whose vision is to unlock the potential of medicinal cannabis and increase both availability and affordability for patients in need. He has also been the Chair of the Board of the Nationaal MS Fonds since 2015.
Samantha Nickerson, Country Lead UK and Ireland at Camurus
Samantha brings over 19 years of industry experience with an established track record in commercialising specialty pharmaceuticals. At Camurus, Samantha leads a multi-functional team and is responsible for all commercial activities in the UK & Ireland.
Her expertise and success in product launch and gaining market penetration in the UK has helped achieving commercial success for Camurus Ltd as it launched its revolutionary treatment, Buvidal® for opioid dependence. Samantha will lead a multi-functional team and be responsible for all commercial activities in the UK & Ireland.
Prior to this, Samantha has spent time in many prestigious companies including Baxter and Baxalta, CR Bard, Smith & Nephew, and Bristol-Myers Squibb.
Sebastian Hallworth, Head of ADMET Services at Concept Life Sciences
With 20+ years of experience, Sebastian brings in-depth knowledge and hands on proficiency of in vitro DMPK studies, design validation and implementation. He is a leading expert at managing development of cost-effective robust assays, bioanalysis, aseptic cell culture, contract research organisation workflows to tight data turnaround times, sample management tracking, and outsourcing.
Sebastian has now been the Head of ADMET Services at Concept Life Science for over 13 years and prior to that he was Senior DMPK for almost 9 years at AstraZeneca,
Simon Collis, Director, Global Commercial and Business Development at Britannia Pharmaceuticals Ltd
A dynamic and inspirational executive, Simon has over 25 years’ experience in a variety of senior strategic commercial, business development and marketing roles within the pharmaceutical and healthcare sectors. He has an entrepreneurial reputation for driving strategic business growth through innovative product marketing and sales strategies and M&A activity. Simon has worked for every size of company, from very large multi-national corporations, such as Schering-Plough, Ipsen and Merck Serono, to new private and equity backed start-up entities, such as Denfleet (now Merz) and Crawford Healthcare, making him adept at adding value and driving sales growth in small and medium-sized businesses (SMEs) by devising and managing market access, pricing strategy, and market segmentation. Simon has also worked in a wide variety of therapeutic areas, including Oncology, Neurology, Mental Health and Addiction.
Tim Watts, CEO at Shield Therapeutics
Tim Watts is currently in non-exec and advisory roles, including NED of Fusion Antibodies PLC. Former biotech CFO in both PLC (Oxford BioMedica, OXB) and private equity backed (Archimedes Pharma) companies and extensive experience in Big Pharma (AstraZeneca).
He has gained comprehensive general management experience through membership of senior executive teams at OXB (2012-2017) and Archimedes (2007-2011), and AZ International Sales & Marketing Organisation (1999-2002).
He also has great experience of PLC board as director of OXB PLC and broader governance and risk management experience in public and private companies gained at Archimedes and as Group Financial Controller at AZ (2002-2006).
Yann Guivarch, Senior Consultant Project Lead chez BELPAEME CONSEIL
Yann has over 20 years of experience in management and executive administration of health and research establishments, public and private. He has a wide curriculum of high-level positions among which Health financing project manager at the Institut National du Cancer, Secretary General at CARPEM, and Finance and Administration Manager for Clinical Research at The Georges Pompidou European Hospital.
Yann holds two masters, one in Health Economic Management at the Université Paris Dauphine, and one in International Relations at Université Paris 1 Panthéon-Sorbonne.
Yann joined Belpaeme Conseil in March 2020 and is involved in the various stages of change management: facilitation of social dialogue, organizational audits, individual support for executives and procedures for quality of life at work. Prior to this he acted as the Deputy Director of the Institut Curie Research Centre.
Jordi Clarimon, Biofluid Lead Specialist. Translational Biomarkers and Imaging Group. Experimental Medicine Department at Lundbeck A/S
For 15 years, Dr. Jordi Clarimon was the principal investigator of the Genetics of Neurodegenerative Diseases Unit (GNDU). He received his degree in Biological Sciences from the University of Barcelona and his Ph.D. from the University Pompeu Fabra. Dr. Clarimón spent 2 and a half years as a postdoctoral researcher at the Laboratory of Neurogenetics, located at the National Institutes of Health (Bethesda, Maryland, USA), and headed by Dr. John Hardy. In 2009 he became the PI of the GNDU. His research focused on the genetic causes related to a broad range of neurodegenerative disorders, including Alzheimer’s disease and other dementias, as well as neuromuscular and movement disorders.
A highly regarded scientist, he now works as Biofluid Specialist in Neurological Disorders for Lundbeck A/S.
Carsten von Blohn, BPL Bioproduct Laboratory GmbH Interim General Manager, PlanB GmbH Founder and General Manager, Speaker of Expert Group Healthcare in DDIM and Member Management Board of Healthcare Shapers.
With a starred collection of managerial roles paving his careers in the field of Rare Disease/Orphan Disease/Biotech businesses in Germany, Mr von Blohn is a result-oriented marketing expert, sales & commercial consultant, and interim manager in the pharmaceutical industry, with an extensive background supporting German affiliates in the set-up and brands in (re)-launch phase.
He heads the PlanB GmbH, a management and consultancy firm dedicated to providing state-of-the-art strategic, operational & interim support companies in the life science industry.
He is also the Speaker of Expert Group Healthcare at Dachgesellschaft Deutsches Interim Management.
“My motto is “done is better than perfect: implement pragmatically, be decisive, think broadly, develop talents, aspire high.”
Title font
Body font
Ahmed Al-Derzi, CEO and Board Member at Consilient Health
Ahmed has over 25 years’ experience in the pharmaceutical industry and is one of the Co-Founders of Consilient Health. At the outset, Ahmed was the company’s Director of Planning and Business Development and played an instrumental part in securing products and strategic partnerships with reputable developers and manufacturers across the globe. In 2011, Ahmed was appointed CEO however he continues to lead the BD teams and devotes significant time to Consilient Health’s BD effort. Ahmed serves on the boards of Medicines for Europe and the British Generic Manufacturers’ Association.
Prior to this, Ahmed spent 10 years at Regent-GM Laboratories in two roles; firstly, Technology Transfer Executive and secondly as Head of Outsourcing, where he was responsible for the in-licensing of new products for the company as well as re-introducing numerous products back into the portfolio through the use of third party CMOs in Europe, India and MENA.
Ahmed is a Chartered Chemist and is currently reading for his Executive MBA at Imperial College London. He also completed his training for designation as a Qualified Person (in the EU) at David Begg Associates in 2003.
Amir Inamadar, Chief Medical Officer at Cybin
A physician with with specialist training in Psychiatry and Pharmaceutical Medicine, Amir brings over 20 years of clinical experience with 15+ years of pharmaceutical industry experience in Drug Development/ Drug Discovery, in both early and late phases.
Amir has been recently appointed as Cybin’s Chief Medical Director. Most recently, he covered the position of Senior Medical Director of R&D Biopharmaceuticals, Neurosciences at AstraZeneca. Prior to this, he worked at Takeda, and GSK. Amir holds a diploma in Pharmaceutical Medicine from the University of Cardiff, and a Medical Degree with a residency in Psychiatry and a junior residency in Nephrology from Nagpur University.
Dr Angelico Carta, President, MD and Co-Founder of Worldwide Clinical Trials
Dr Carta has more than 20 years’ experience in the international pharmaceutical industry and CRO management. Early in his career, Dr Carta’s leadership experience included a position as Senior Director and Head of Clinical Research within a large global pharmaceutical company, encompassing Phase II–III trials across CNS, cardiovascular, and endocrinology. Dr Carta’s vision, combined with that of three like-minded individuals of long association, Dr Neal Cutler, Dr Neil Kurtz, and Dr Michael Murphy, led to the founding of Worldwide Clinical Trials in the mid-1990s. Within four years under Dr Carta’s direction, the growth of ex-US operations into a multimillion-dollar enterprise spanned 25 countries.
Dr Carta also served for four years as President of Global Business Initiatives for a global pharmaceutical company. Subsequently, he became Managing Director of a private pharmaceutical development consultancy specializing in CNS drug development services, with multiple global pharmaceuticals and CRO customers to its credit.
Dr Carta is the first co-author of over 30 papers on clinical neuropsychopharmacology published in internationally renowned journals. Additionally, he has co-authored six reference texts on the clinical development of Alzheimer’s disease, antipsychotic drugs, and CNS drug development. Dr Carta continues to present and review clinical data at numerous clinical neurology and neuropsychiatry meetings internationally. He is a board-certified neurologist and holds an M.D. from the University of Rome, School of Medicine, with training in neurology from King’s College Hospital in London.
Dr Christoffer Jensen, Head of Neurology, Medical Strategy and Communication at Lundbeck
A trained Medical Doctor, Christoffer has a background in business and communications and has spent the last 10 years of his career in Medical Affairs operating at a local and regional level. Within Medical Affairs he has focussed predominately in Neurology, with significant work on Multiple Sclerosis and Pain management. Christoffer is now Head of Neurology, Medical Strategy and Communication at Lundbeck. Prior to this, he was Nordics Medical Affairs Director at Akcea, where he focussed on innovative therapies.
Beforehand, he has been Medical Director at GSK Denmark and Iceland, Nordic Medical Director for Grunenthal, Medical Director for Biogen Denmark and Iceland, and Head of Business Development/Chief Operating Officer at IPC Plc (now Azanta).
Christoffer has left clinical practice for several years but his enduring commitment to improving the lives of patients is clearly demonstrated by the steps he’s taken in the corporate world.
David Impey, Partner at Saiga Life Sciences Capital
David has worked in Pharmaceutical Sales and Marketing for over 30 years, the last 15 of which have been in International Marketing. An expert at new product launches, he has extensive experience of managing marketing and multi-disciplinary project teams, working on products such as Aricept for Alzheimer's Disease and Omnic/Flomax for BPH as well as the pre-launch development of Vesicare for overactive bladder.
Starting as a medical representative for Pfizer, he then continued his career as Product Manager for Carlo Erba, Regional Business Manager for Pharmacia, Senior Product Manager and European Brand Director for Yamanouchi Europe. He then worked for Eisai Europe as European Director of Marketing and as COO for Taran Pharma Europe.
He is now in business for himself, offering strategic marketing and market access services to companies and/or their investors who are in the early stages of their life cycle and who are looking to establish a solid commercial base from which to build.
David is a Fellow of the Chartered Institute of Marketing and a Member of the Interim Managers Association, Director at BigBear communications and Partner at Saiga Life Sciences Capital.
“I am proud to have worked in the industry for 30-something(!) years. Success, for me, is measured by how much one can change attitudes and behaviours in the medical community, the industry and patients. For an industry that has lost sight of this, I say: ‘Be Bold; Be Fearless.’ Our customers will love us for our leadership.”
Giuseppe Mazza, PhD. Co-Founder and CEO at Engitix
Dr Giuseppe Mazza co-founded Engitix and has served as Chief Executive Officer (CEO) since the Company’s formation in 2016. His mission is to understand the role of human microenvironment (“ECM”) in driving disease progression to develop novel therapies for patients affected by advanced fibrosis and solid tumours
Engitix is built on Dr Mazza’s pioneering research, where he developed novel decellularisation procedures for human tissues, including whole livers, while working in the laboratory of Professor Massimo Pinzani at the Institute for Liver and Digestive Health (IDLH), Division of Medicine, University College London (UCL).
Dr Mazza was a Post-Doctoral Fellow at the UCL IDLH, where he was also responsible for operating the Tissue Engineering and Regenerative Medicine Laboratory. Dr Mazza received his PhD in Tissue Engineering and Organ Regeneration from UCL. Dr Mazza was Director R&D at Promethera Bioscience from 2015 to 2017.
He has been involved in several international healthcare organisations, including serving on the Steering Committee of the non-profit European Haemophilia Consortium (EHC). In 2011, he founded the healthcare charity Associazione Icore ONLUS, where he served as President for six years.
Graham Mullis, Group CEO at Novacyt
Graham was appointed CEO of Novacyt in 2014, bringing with him over 30 years in healthcare, pharmaceuticals, and medical device markets. He is an internationally experienced leader with multi-disciplinary background and has lead multiple successful exits including Biocompatibles Eyecare, ClearLab, VisionTec and Optivue. Graham is a C-level executive with FTSE 250 (Biocompatibles International) and NASDAQ (1-800 CONTACTS).
“It has been some time since the Life Science and Healthcare sectors around the world have been challenged by something as significant as the COVID-19 pandemic which hit some 20 months ago. I am privileged to have led and worked with an exceptional team at Novacyt, an emerging diagnostics business that demonstrated its capabilities with the launch of the world’s first CE-IVD for COVID-19. We look forward to the end of the pandemic when we can focus on continuing to make a difference to people’s lives around the world in other clinical areas.”
Holger Montag, Country Head at Micro Labs
Holger has 15 years of experience in the pharmaceutical industry. He started his career in 2006 as a project manager at Mylan dura and transferred to Bluefish Pharmaceuticals as a product manager in 2010. He joined Micro Labs in 2014, first as Marketing Manager and since 2016 as Country Head Germany.
Dr Ian Hudson, OBE, Senior Advisor for Integrated Development at The Bill and Melinda Gates Foundation
Working with the Bill and Melinda Gates Foundation, Dr Ian Hudson has a leading role on the team in areas that include optimising clinical studies and strengthening regulatory systems in Africa and other low-resource regions, particularly for malaria, polio, and COVID-19 drugs.
Ian was a practicing paediatrician before joining SmithKline Beecham in 1989 to work in research and development. In 2001, he joined the UK government’s Medicines & Healthcare products Regulatory Service (MHRA), where he served as director of licensing and then CEO. Ian was the UK delegate to the scientific committee of the European Medicines Agency’s Management Board, CHMP, later becoming its Vice Chair. He was also an honorary senior lecturer in clinical pharmacology at the University of London and served as Chair of the International Coalition of Medicines Regulatory Authorities.
Ian earned his medical degree, with a specialty in neonatal haematology, from London Hospital Medical College, and has postgraduate qualifications in paediatrics and pharmaceutical medicine. In 2020, he was awarded an OBE for Services to Healthcare, received a Faculty of Pharmaceutical Medicine President’s medal for contribution to pharmaceutical medicine, and was named a Global Fellow in Medicines Development by the International Federation of Associations of Pharmaceutical Physicians and Pharmaceutical Medicine.
Jasna Knezevic, Consultant in Pharmacovigilance
Starting as an Assistant Professor and specialist paediatrician for the University of Zagreb Medical School and Hospital, in 1995 Jasna moved to GSK where she spent 17 years, building a starred curriculum, and ultimately holding the position of Medical Director. Focussing on Global Clinical Safety and Pharmacovigilance, Jasna held the position for over 11 years, leading and training safety physicians and curating the CNS portfolio, as well and the pharmacovigilance for many other products.
She is now a private consultant in pharmacovigilance, safety, and risk assessment. Here at RSA, we have also had the pleasure of working with her, when she covered the position of Interim Medical PV Consultant.
Jina Swartz, Executive MD at MSD.
Dr Jina Swartz trained in Internal Medicine and Neurology in South Africa, where she obtained her MBBCh (MD) degree cum laude, followed by an MSc Medicine (in Neurology). Within Neurology, she has a special interest in neurodegenerative disorders (Alzheimer’s disease and related dementias, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, spinocerebellar degenerations), cerebrovascular disease, infectious neurology, epilepsy, pain and migraine, paediatric neurology and neurogenetics. Following the award of a post-doctoral research fellowship in 1998, she completed a PhD at the University of Cambridge at the Cambridge Institute for Medical Research, exploring molecular mechanisms of neurodegeneration in cellular models of Huntington's disease and trinucleotide disorders. She was also recently appointed to the Fellowship of the prestigious Academy of Medical Sciences in the UK.
Jina’s experience in clinical development spans almost twenty years in industry. Dr Swartz worked in Exploratory Medicine/Translational Medicine in the Neurology CEDD at GlaxoSmithKline. She was then Senior Medical Director in CNS at Eisai Global Clinical Development. In 2010 she became Neurology Therapeutic Area Leader (Vice President) at Bracket/Signant. In 2014, she worked as Senior Medical Director in the CNS Therapeutic Area Unit at Takeda. Her current role is Therapeutic Area Head Neuroscience and Respiratory/Immunology, Executive Medical Director in Global Clinical Development, based in Europe, at Merck Sharp and Dohme (Merck and Co), as an international expert in developing therapies for neurological disorders and chronic cough. Over this time frame, she has worked in increasing roles of responsibility within both Translational Medicine and Clinical Development, focussing on Alzheimer’s disease, dementias and disorders of cognition, Parkinson’s disease, multiple sclerosis, pain, epilepsy, cerebrovascular disease, schizophrenia, Huntington’s disease, spinocerebellar ataxias, Friedreich’s ataxia, Duchenne’s muscular dystrophy, Down syndrome, sleep disorders and refractory chronic cough.
Dr Swartz previously performed sessions at the Medical Foundation for Care of Victims of Torture, in London. She is also an Honorary Consultant at Imperial College London, where she performed clinical sessions at the West London Cognitive Disorders Treatment and Research Unit and is currently a visiting Lecturer on the MSc Pharmacology course at Kings College London. She also serves as an Industry Board Member at Ataxia UK and an External Advisory Board Member for Sheffield Translational Neuroscience Centre for Chronic Neurological Disorders.
Jo-Hanna Krause, Head of Marketing at Micro Labs
Jo-Hanna has several years of experience in marketing & sales of prescription RX medicines and OTC products combined with a successfully completed Master of Science degree. She started her career during her dual studies at G. POHL-BOSKAMP GmbH in Germany. In 2016, she joined MEDAC GmbH as a product manager in the field of rheumatology and dermatology. This was followed by a position at TAD GmbH as Brand Manager in the field of cardiology and neurology before she became Head of Marketing at our client Micro Labs Ltd. at the beginning of this year.
John Bolodeoku, Clinical Director/Chief Medical Officer at Zeab Therapeutic Limited
Dr Bolodeoku completed his MBBS in 1986 from the University of Ibadan, Nigeria. He then started his post-graduate medical career as a registrar in Chemical Pathology at St Helier Hospital, Carshalton, and St Georges Hospital Medical School, London. He then went off to pursue his doctorate training taking a position at Nuffield Department of Pathology at the John Radcliffe Hospital, Oxford, and subsequently Senior Registrar/Clinical Lecturer at the Department of Chemical Pathology and Human Metabolism at Royal Free Medical School, London. He completed his training in Chemical Pathology and was awarded his completion of specialist training (UK-CCST) in 1998 and is a Fellow of the Royal College of Pathologists. He is an Honorary Consultant Physician at North Hampshire Hospital Hampshire in Cardiology (Lipids), has an MSc in Clinical Biochemistry from the University of London, a DPhil from Keble College, Oxford, and an MBA from the University of Liverpool.
After completing his specialist training, he decided to venture into the Pharmaceutical Industry, starting as a Clinical Research Physician in BIOS - a Consulting and Contract Research Organisation. He subsequently worked for Pfizer, Astellas, Daichi Sankyo, Roche, Biogen, ICON, Lilly, and as Executive Country Medical Director and Compliance Lead, Celgene UK & Ireland. In May 2020, he returned to his consulting and marketing company, JB Consulting MDP Limited. Currently he is also Chief Medical Officer at Innoture Medical Technology, Zeab Therapeutic, Covid-19 Health Clinics, and Affinity Biomarker, as well as being Clinical Director & Chemical Pathologist at PathDirect and Chemical Pathologist at London Medical Laboratory.
Kok Eng Ng, Director of Human Resources at Duke-NUS Graduate Medical School
A skilled professional in the field of Human Resources for Academia, Kok Eng has built and impressive career in employment recruiting and management.
Graduated from the Curtin University of Technology, he served some time in the Republic of Singapore Air Force before moving on to Seagate Technology International in their HR department.
He then became HR manager for Applied Materials before moving to Duke-NUS where he started as Senior Manager.
Kok Eng has recently celebrated a decade at Duke-NUS being Deputy Director and then Director of HR for the establishment.
Dr. Kulasiri Gunawardena, Director at KG PharmaConsulting Ltd & Director translational Medicine Expert at Novartis
A widely respected physician trained in Respiratory/General Medicine to specialist level, Kulasiri has extensive experience in clinical research and pharmaceutical industry experience of over 20 years, working within the CRO and big pharma environments. He is now running his own consulting business, PharmaConsulting Ltd.
Prior to this, he has been Director of Translational Medicine Discovery & Profiling at Novartis and Senior Research Physician and Director for Respiratory & Inflammation Therapy Area at AstraZeneca, as well as covering many other roles including both in the corporate field and academia.
Lee Smith, Principal Consultant & Managing Director at GreyRigge Associates Ltd.
Dr Lee Smith, the principal consultant and managing director of GRA for GreyRigge, has a passion for biotech & data driven results but above all, helping people and companies achieve their objectives and grow. He has been working in the biotech area for over 20 years and has worked with large multinational organisations, small & medium sized enterprises (SMEs) as well as with virtual start-up biotech companies. His experience spans biopharmaceutical CMC, process, analytical, formulation pre-clinical and clinical assay development as well as experience in product characterisation and regulatory submissions and interactions. His focus encompasses R&D, product development, pre-clinical & clinical development, formulation, program management, business development and Sales & Marketing. Lee supports both early-stage companies through to multinational corporations adding both commercial, operational and technical expertise.
Malcolm Taylor, Vice-President of Manufacturing and Supply at Norgine
A successful and experienced manager, Malcolm Taylor has built an impressive career, which spans well over three decades.
Currently acting as Vice-President of Manufacturing and Supply at Norgine. Prior to this Malcolm has been Managing Director at Arca Management, and Vice-President of Operational Excellence at Cardinal Health Europe. Beforehand, he has held the position of Managing Director for Mitchell Cotts Chemicals, and Commercial Director of Synthetic Chemicals for Shell Chemicals.
Dr. Manfred Kurz, Associate Director of Regulatory Affairs and Product Lead at EUSA Pharma
Widely respected in his field, Manfred has dedicated his career to Regulatory Affairs. Before his appointment at EUSA Pharma, he worked for BeiGene, Lundbeck, Eisai EMEA, and Mylan, a major generics company based in Switzerland as Senior Global Regulatory Affairs Manager in February 2011. Prior to that, between 2008 and January 2011, he was Regulatory Affairs Manager at BioGeneriX AG/ratiopharm (TEVA) in Mannheim (Germany) managing regulatory activities associated with the development of investigational biosimilar/bio better proteins.
From 2004 – 2008 he served as Regulatory Affairs Associate in the Product Lifecycle division at CSL Behring AG, Bern (Switzerland) where he was responsible for assigned marketed human plasma products. Before that he worked in the regulatory affairs department at Chiron Vaccines, Marburg (Germany).
He holds a master’s and doctoral degree in molecular cell biology from University of Goettingen (Germany) and did postgraduate research at the University of California at San Francisco (UCSF, USA). He has also accomplished a postgraduate qualification in Drug Regulatory Affairs at University of Bonn (Germany).
Matthew Lumley, Senior Director Rare Diseases and Clinical Development at Moderna
A medicine graduate from Imperial College London, Matthew quickly collected an array of postgraduate qualifications, among which a PhD in Cardiovascular Physiology and Clinical Research at King’s College London, before egregiously stepping into the corporate world.
Starting off as an Honorary Consultant Physician for ORLA Healthcare, he then moved to Pfizer where he spent 3 years, culminating with his appointment as Medical Director for Rare Diseases.
He is now Senior Director of Rare Diseases and Clinical Development at Moderna.
Dr Maziar Assadi Gehr, Medical Director of Anti-Infectives at Basilea Pharmaceutica
An accomplished Medical Doctor with work experience in clinics, academia, and 14 years in the pharmaceutical industry, Maziar is now Medical Director of Anti-Infectives at Basilea Pharmaceutica. A PhD specialised in DMD (Duchenne muscular dystrophy), Maziar began his corporate journey with Roche, starting off as Senior Scientist and ending as Medical Manager. He then moved to Actelion, acting as Global Medical Affairs Physician. During his occupational career, Maziar has been recognised for his in-depth knowledge of the pharmaceutical value chain applied to different kinds of pathologies and is also experienced in activities linked to business development and CE-marking.
.
Dr Mustapha-Kamal Benguerba, Global Executive Director, Real World Evidence at Novartis Gene Therapies
Starting as a medical doctor specialised in Geriatric Oncology, Dr Benguerba has worked as a clinical researcher for three world-leading universities before moving to the corporate world in 2004, when he started as an International Marketing Manager for Sanofi (then Aventis).
This opened the door to many Scientific Advisor roles, when he worked for Europharma for Life Science, Terre Neuve, Roche, AstraZeneca, Norgine, Ferring Pharmaceutical, and Verte Pharmaceuticals.
He then moved on to Medical Director roles, working at PTC Therapeutics, and Spark Therapeutics.
Dr Benguerba is now Global Executive Director of real World Evidence at Novartis, as well as being an external lecturer for the Universite de Paris.
A Global Medical Affairs Executive Director with 17 years of passionate experience in biotechnology & pharma industry spanning across multiple functions in a matrix environment. Mustapha spent 9 years in rare diseases and more than 3 years in Gene Therapies in a highly competitive environment.
.
Nick Rhys-Jones, SVP, Pharmaceutical/Biotech Leader
Nick carries with him 25 years of pharmaceutical and healthcare industry experience in a wide range of Commercial Leadership roles at local, regional, and global level. Throughout his career he established a good track record of leading and developing international cross-functional teams, country builds and General Managers in Oncology, Orphan and CV disease areas.
Most recently, Nick was Head of Commercial Operations at EUSA Pharma. Prior to this he worked for 10 years at Daiichi Sankyo Europe covering a variety of roles throughout the years, and as director of Morpheus Health Ltd, digital health start up developing evidence based on-line solutions for sleep, stress, and anxiety disorders.
Piers Morgan, CFO at COMPASS Pathways plc
An experienced biotech executive and finance leader, Piers qualified as a Chartered Accountant in PwC’s London office before spending almost 10 years as an investment banker, focusing on Mergers, Acquisitions and Financings, initially at Close Brothers and later in Ernst & Young’s Lead Advisory practice.
In 2000 he joined Arrow Therapeutics (sold to AstraZeneca for $150m) before working as CFO for a number of other biotech companies in UK, France, Netherlands, and Ireland. He has led IPOs on Nasdaq (uniQure: QURE and Verona: VRNA), as well as on AIM and Euronext Paris, and has raised more than $400m in new equity, convertibles, venture debt and strategic big-pharma investments. He has also been involved in numerous licensing transactions and the sale of Quethera to Astellas for £85m.
Piers holds an MA in Law and Management Studies from Cambridge University.
Dr Riccardo Palmisano, President of Assobiotec
Riccardo Palmisano is the president of Assobiotec, the Italian Association for the Development of Biotechnology.
After graduating with a degree in medicine, Riccardo moved away from a conventional medical career path. He took his first job in commercial marketing with Farmitalia Carlo Erba, at that time the largest R&D pharmaceutical company in Italy. After broadening his skill set, also through training high potential young professionals at Bocconi University, he moved to Menarini, an established Italian pharmaceutical company. After a rapid career in the parent company, he was appointed as General Manager of one of the Italian companies of the Menarini Group, Lusofarmaco, in 1995. Following a short additional period with Menarini’s head office as head of all Italian operations, he moved to Shire (now part of Takeda), originally a small UK-based pharmaceutical company, at a crucial stage in its expansion into the four largest European markets and grew the Italian branch from scratch to become a market leader.
Riccardo has also had leading roles at high-profile pharmaceutical companies including GSK, where he served as vice president, Genzyme as VP, Managing Director and GM for Italy, and finally Molmed, as CEO of the listed cutting-edge cell & gene company. After an impressive 36 years in the industry, he currently sits on the board of multiple international biopharmaceutical organisations and is a highly respected board name in the biopharmaceutical industry.
Dr Segundo Mariz, Scientific Administrator at EMA
A scientific administrator of international esteem, Dr Mariz has been working as Pharmaceutical Physician since 1990, when he started his career as Medical Advisor at UCB. Ending that chapter of his career in 1998 after holding the position of Medical Director for 5 years, Dr Mariz continued his professional journey working as Medical Manager and Head of Diabetes for Takeda.
Segundo is now Scientific Administrator for the European Medicines Agency, where he has been involved in following Orphan Drug Designations for the Orphan Drug Section in the EMA for over 10 years.
Prior to this, he served as Senior Medical Assessor and Deputy Unit Manager at the MHRA for over 4 years.
Simon Higginbotham, Chief Marketing Officer at Parexel
“I am delighted to have spent my career in the Life Science and Healthcare industry and to have worked with amazing people all over the world. I am fortunate to have served in a Chief Marketing Officer role in pharmaceutical services four times and gained experience as president of a mid-tier CRO and serving on the board of ACRO during my time. Today I am Chief Marketing Officer of Parexel and love the way that our industry has pivoted to the patient. In my spare time, I am a practicing paramedic and get to see the challenges in healthcare first-hand.
Congratulations to the RSA team on being such an amazing partner to our industry leaders and for this 40th-anniversary milestone!”
Steve Bates, CEO at BioIndustry Association
Steve Bates has been the CEO of the UK Bioindustry Association since 2012. He currently chairs the International Council of Biotech Associations and has been a Board member of Europabio since 2015. Steve is the visible face of the vibrant UK life sciences industry to government and media. He sits on the UK’s Life Sciences Council and Life Sciences Industrial Strategy Implementation Board. Steve has championed with government effective industrial incentives like the Biomedical Cataylst which have crowded-in private sector investment into UK SMES. He has forged links for the sector across the USA, Europe and in China. In his time at the BIA Steve has developed new member groups focused on cell and gene therapy, genomics and engineered biology. A strong advocate of partnership working, Steve champions sector collaboration with research charities and academia. Proud to lead an organisation with a diverse Board with over 40% female representation, Steve is committed to next generation talent and developing the skills needed for the sector to flourish. Before the BIA, Steve worked for Genzyme and as an advisor to the UK Government of Tony Blair. He was made OBE for services to innovation in 2017.
Stuart Paynter, Chief Financial Officer at Oxford Biomedica
Stuart Paynter joined Oxford Biomedica and the Board in August 2017. Mr Paynter has 17 years’ experience in the pharmaceutical and healthcare sectors. He qualified as a chartered accountant with Haines Watts before moving to EDS. He subsequently joined Steris and worked in a variety of roles within the healthcare and life sciences divisions prior to becoming the European Finance Director. Mr Paynter moved to Shire Pharmaceuticals where he became the Senior Director of Finance Business Partnering for all business outside of the US. He then moved to a corporate finance role before becoming the Global Head of Internal Audit. Prior to joining Oxford Biomedica Mr Paynter was Head of Finance Business Partnering at De La Rue plc. He is a member of the Institute of Chartered Accountants in England and Wales.
Stuart Rose, Owner and CEO at MiViVa
With over 35 years of experience in domestic and international pharmaceuticals, Stuart Rose has worked for some of the world’s largest, (and smallest) businesses in sales, marketing, and commercial leadership roles.
He is now owner of MiViVa, a specialist consultancy to support strategy & culture creation in healthcare & interim support to industry. His experience has given him insights into the things that leaders of businesses need to get right to create longevity and success.
Prior to this, he has been MD for UK and Ireland at Merz Pharma, as well as working for Oxford Immunotec, pharmaSMART, Elan, GSK, and Novo Nordisk.
Stuart has also devoted last year to writing, culminating with the production of his first manuscript
Ted Smith, MD at UKHR Ltd
Ted Smith is a senior human resource and development consultant, and owner of UKHR.com Ltd. He holds a degree in Environmental Sciences, and a diploma in HRM.
Ted has held positions at Glaxo, Nielsen, Vernalis, the Medical Research Council and Wellcome Trust. He’s worked in Europe, USA, Africa, and Asia at executive and board level, and is currently chair of the charity Ideas Foundation. He was also a trustee at Wysing Arts and was the Chair at Herts Careers.
Among his many accomplishments, Ted is the author of the Train Blog: Odd and Weird People-Watching (2020), and “Human Resources A to Z: A Practical Field Guide for People Managers”. He also published several children’s stories for charity, including The Exploding Turnips, Arthur Ramsbottom, and the “Dinkle Donkle” and “Willie the Hippo” series, all supporting NHS, Save the Children and Macmillan Cancer Support.
Dr Melanie Lee, CEO at LifeArc
Prior to joining LifeArc as CEO, Melanie pursued a 30-year career in healthcare R&D and gained leadership experience both from the biopharmaceutical industry and from the medical research charity sector. Melanie’s previous roles have included Chief Scientific Officer at BTG (most recently) as well as senior positions at GSK, Celltech and UCB. She has previously held Trustee appointments at Cancer Research Technology and Cancer Research UK and currently serves on the Board of Directors of Sanofi, as well as being an NED for NightstaRx, a company focussed on developing gene therapy treatment of Choroideremia to prevent the deterioration of eyesight in patients with this X-linked disease.
Thomas A. Weaver, CEO at PetMedix
Tom enjoys creating value in new projects, particularly early-stage companies. Previous experiences include being a founding scientist in Hexagen Genetics LTD (acquired by Incyte PLC), Geneservice Limited (acquired by Source Bioscience PLC), and Congenica LTD, Next Gen Diagnostics LLC and PetMedix Ltd (still private). He has more than 20 years of experience in technology development, specializing in genomic based research and drug discovery, as well as commercial expertise in building and managing multifunctional teams and developing business in the EU, USA and China.
Previous academic and government science experience includes having been a Medical Research Council (MRC) Director in Oxford where he managed the UK’s largest mammalian genetic animal R&D infrastructure and was a founding member of the International Mouse Phenotyping Consortium (IMPC), and before that he set up and managed a national genomic service program (part of the MRC human genome project, Hinxton, UK). He received his BSc in both mathematics and biochemistry (University of Wisconsin, Madison), and a PhD in experimental oncology (McArdle Labs, University of Wisconsin Medical School) before moving to the UK where he carried out post-doctoral research at the MRC Lab for Molecular Biology and the University of Cambridge.
Helen Stephenson-Ellis, Chief People Officer at Oxford Biomedica
Helen Stephenson-Ellis joined Oxford Biomedica in April 2018. She brings 20 years’ experience in senior Human Resources roles within the Biopharmaceutical sector, including a number of years in various HR Business Partnering roles at GSK. Following AstraZeneca’s acquisition of MedImmune, she moved to Cambridge UK to head up HR for MedImmune’s site, and proceeding this became Global HR Director for AstraZeneca. Prior to joining Oxford Biomedica, she was Group Human Resources Director for Vernalis plc, leading HR across Vernalis’ UK and US sites. She holds a BA (Hons) degree from Northumbria University in the UK and is a member of the Chartered Institute of Personnel and Development.
Baroness Susan Greenfield, CEO and CO-Founder at Neuro-Bio Ltd.
“I’ve never thought of my career as work and was always surprised that I was being paid to do something I enjoy enormously!”
Susan Greenfield is a research neuroscientist, and author based in Oxford. She has held research fellowships in the Department of Physiology Oxford, the College de France Paris, and NYU Medical Center New York. She has since been awarded 32 Honorary Degrees from numerous universities worldwide. In 2000 she was elected to an Honorary Fellowship of the Royal College of Physicians. Further international recognition of her work includes the ‘Golden Plate Award’ (2003) from the Academy of Achievement, Washington USA, the L’Ordre National de la Légion d’Honneur (2003), from the French Government, and the 2010 Australian Medical Research Society Medal. She has held a Visiting Professorship at the Medical School, University of Melbourne, Australia and is currently CEO of a biotech company (www.neuro-bio.com) that she founded in 2013.
Clive Dix, PhD, Chief Executive Officer at Medicine Discovery Catapult
Clive has more than 30 years’ experience in life science research, with over 20 years in senior pharmaceutical industry positions and a degree and PhD in Pharmacology. His expertise includes an in-depth understanding of all facets of drug discovery and development, a broad knowledge of the scientific and commercial landscape of a variety of therapeutic areas and solid experience of the pharmaceutical business and finance community supporting the sector.
Clive was co-founder and chief executive of Convergence Pharmaceuticals Ltd, which was acquired by Biogen in January 2015.
Previously, Clive was co-founder and chief executive of PowderMed Ltd, a vaccines development company acquired by Pfizer in November 2006. Prior, he was senior vice president, research and development and a Board member of PowderJect Pharmaceuticals plc, until its acquisition by Chiron Vaccines in 2003. Clive began his career in industry at Ciba-Geigy, and shortly after he joined GlaxoWellcome, where he attained the position of UK research director in 2001. Clive is a recent past chairman of the BioIndustry Association, is currently sits on the boards of Touchlight Genetics Ltd and Centauri Ltd as a non-executive chairman, and is a non-executive member of the Medicines Discovery Catapult Board. Clive is currently the Deputy Chair of the UK Vaccine Task Force.
David Picard, CEO and Founder at Moleac
David co-founded Moleac in 2003 and has been serving as CEO since. He is also Independent Director for Greenship Holdings. Prior to founding Moleac, David spent ten years at the Boston Consulting Group (“BCG”) where he was a member of the worldwide Health Care Practice Area. He has worked both in Europe and in Asia. He has extensive experience in working in China and in Korea.
David graduated from the two top engineering schools in France, Ecole Polytechnique and Ecole Nationale Supérieure des Mines de Paris, where he majored in mathematics and economics. David also holds a French degree (“DESS”) in business law.
The RSA Group
The Gate House
Fretherne Road
Welwyn Garden City
Hertfordshire
AL8 6NS
Registered in England as:
RSA Consulting Ltd. Company Number 01803896
and RSA Interims Ltd. Company Number 08433229
© 2025 The RSA Group.
Website designed and built by Happy Giraffe